View Procedure
| Procedure Name | Granting permission to import finished addictive drugs, psychotropic substances, precursor substances with registration numbers for circulation |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address:
Phone:
Email:
|
|
Legal Basis of the Procedure
|
- Law on Pharmacy No. 105/2016/QH13
- Decree 102/2016/ND-CP on Drug business requirements
- Decision 151/2007/QD-TTg dated September 12, 2007 promulgating the Regulation on the Import of Drugs without Registration Numbers in Vietnam
- Circular 19/2014/TT-BYT dated June 02, 2014, management of addictive drugs, psychotropic drugs, and drug precursors
- Circular 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export, import of medicines and packaging in direct contact with medicines
|
|
Processing time
|
- Within 15 working days from the date of reception of valid and sufficient dossier.
|
|
Fee
|
VND 0
|
Required Documents
|
No.
|
Type of Document
|
Remarks
|
|
1
|
Import orders for finished addictive drugs (or psychotropic substances, precursor substances) with registration numbers
|
01 original copy
|
|
2
|
Inventory report of finished addictive drugs (or psychotropic substances, precursor substances)
|
01 original copy
|
Process Steps
|
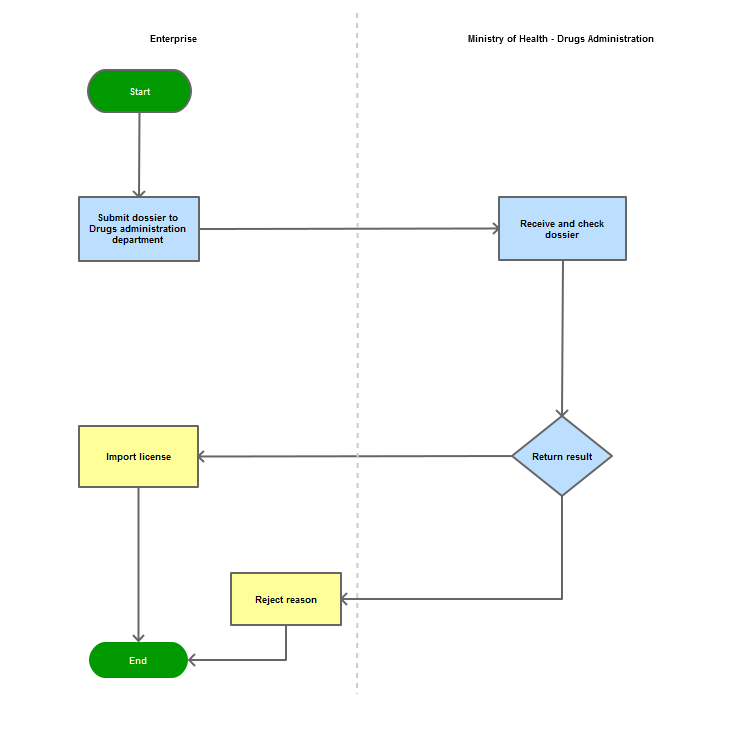
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
The Administration shall inform the applicant of the results. In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map:
|
|---|
| Category | Procedure |
|---|
Please customize this view
This procedure apply to theses Measures.