View Procedure
| Procedure Name | Granting permission to import finished addictive drugs, psychotropic substances, precursor substances without registration numbers for circulation |
|---|
| Description |
|
Category
|
Procedure for licensing
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address:
Phone:
Email:
|
|
Legal basis of the Procedure
|
- Law on Pharmacy No. 105/2016/QH13
- Decree 102/2016/ND-CP
- Decision 151/2007/QD-TTg dated September 12, 2007, promulgating the Regulation on the Import of Drugs without Registration Numbers in Vietnam
- Circular 19/2014/TT-BYT management of addictive drugs, psychotropic drugs, and drug precursors
- Circular 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export/import of drugs/medicines and packaging in direct contact with drugs/medicines
|
|
Processing time
|
- Within 20 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
VND 0
|
Required documents:
|
No
|
Type of documents
|
Note
|
|
1
|
Import order (Form 5a)
|
01 original copy
|
|
2
|
Inventory report of addictive drugs
|
01 original copy
|
|
3
|
Certificate of Pharmaceutical Product (CPP) or domestically-granted Certificate of Free Sale (FSC) or GMP certification (if there are numerous facilities taking part in production, GMP certificates of all these facilities are required)
|
01 original copy
|
|
4
|
Standards and testing methods
|
01 photocopy
|
|
5
|
01 set of original labels enclosed with the user guides in actual circulation in the country of origin; 02 sets of labels intended to be put in circulation in Vietnam enclosed with user guides already translated into Vietnamese.
|
Original copy
|
|
6
|
Pre-clinical and clinical records for drugs containing new pharmaceutical substances, drugs with new combinations of circulating pharmaceutical substances, already translated into English or Vietnamese.
|
01 photocopy
|
Conditions:
|
1
|
a) Drugs composed of pharmaceutical ingredients of groups with pharmacological effects or preparation forms with few registration numbers for circulation in Vietnam according to the list of drugs with registration number for circulation in Vietnam in each period;
b) Drugs composed of pharmaceutical ingredients without registration numbers for circulation in Vietnam:
-Drugs composed of pharmaceutical ingredients with registration number for circulation in Vietnam but at the time of submission of import dossiers, these registration numbers have expired without registration renewal;
- Drugs composed of pharmaceutical ingredients which have been permitted for circulation in other countries but are not yet been granted a registration number in Vietnam, except new pharmaceutical ingredients;
c) Rare drugs, drugs imported for the needs of treatment in special cases or the drugs with special formula for the needs of treatment.
|
|
2
|
Central Pharmaceutical Company 1, Central Pharmaceutical Company 2, Central Pharmaceutical Company 3, SAFACO, YTECO, HAPHARCO are responsible for the supply of addictive ingredients and finished addictive drugs to the sellers and users nationwide.
|
|
3
|
Central Pharmaceutical Company 1, Central Pharmaceutical Company 2, Central Pharmaceutical Company 3, SAFACO, YTECO, HAPHARCO are responsible for the supply of psychotropic substances and precursor substances to the sellers and users nationwide.
|
Process Steps
|
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
The Administration shall inform the applicant of the results.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map: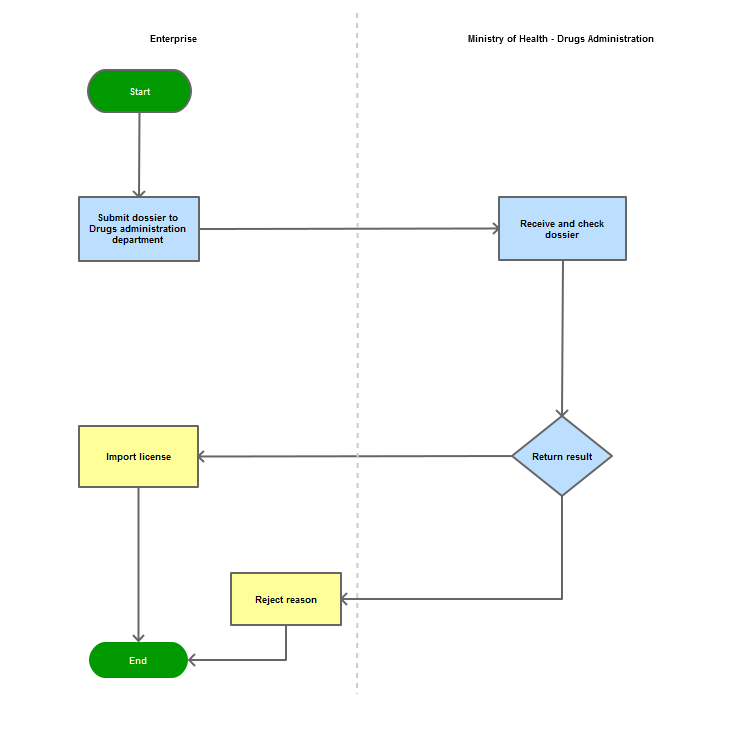
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures


