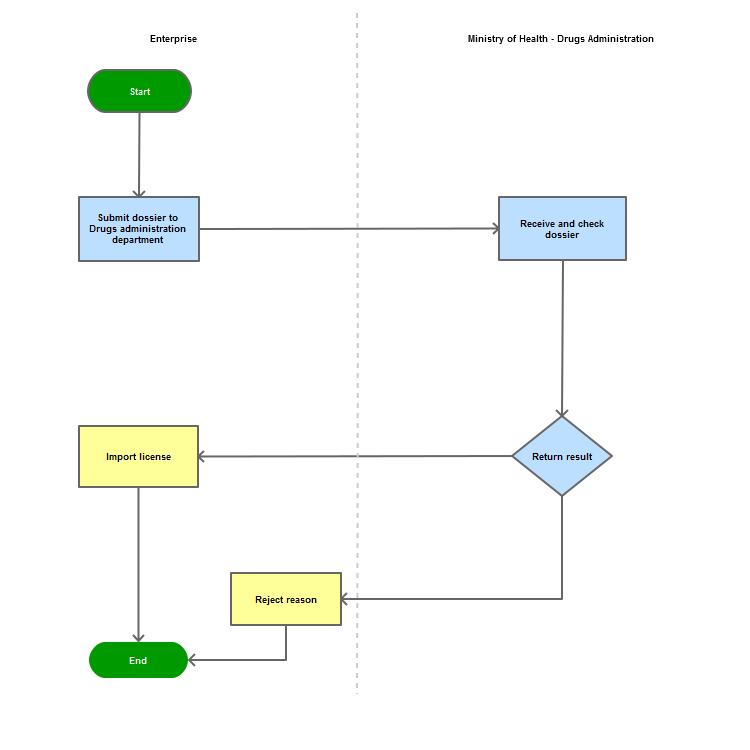
|
The Ministry of Health shall grant the import permit if one of the following conditions are met:
a) Drugs composed of pharmaceutical ingredients of groups with pharmacological effects or preparation forms with few registration numbers for circulation in Vietnam according
to the list of drugs with registration number for circulation in Vietnam in each period;
b) Drugs composed of pharmaceutical ingredients without registration number in Vietnam:
- Drugs composed of pharmaceutical ingredients with registration number for circulation in Vietnam but at the time of submission of import dossiers, these registration numbers have expired without registration renewal;
- Drugs composed of pharmaceutical ingredients which have been permitted for circulation in other countries but are not yet been granted a registration number in Vietnam, except new pharmaceutical ingredients;
Rare drugs, drugs imported for the needs of treatment
in special cases or the drugs with special formula for the needs of treatment.
(Clause 2 Article 7 of Decision No.151/2007/QD-TTg dated September 12, 2007 of the Prime Minister stipulating on the import of drugs/medicines without registration numbers in Vietnam.
|