
Legal Document
Seal of standard conformity and seal of technical-regulation conformity
1. Seal of standard conformity and use of standard conformity seal
The registered certifying organization may prescribe the standard conformity seal including form, structure, and expression and use the standard conformity seal to issue to objects certified standard conformity and must satisfy the following basic requirements:
a) It must clear, not confuse with other seals;
b) It must express full signs of respective standard used as basis for certifying the standard conformity.
In case where organizations or individuals announce the standard conformity on the basis of results assessed by them, it not required to provide for form, structure, expression and the standard conformity seal is not allowed using.
2. Seal of technical-regulation conformity and use of technical-regulation conformity seal
a) The technical-regulation conformity seal has form, size as prescribed in Annex I of this Circular;
b) The technical-regulation conformity seal is used directly on products, goods or packages or in technical documents or on labels stuck on products, goods at apparent and readable positions;
c) The technical-regulation conformity seal must be ensured to not be erased easily and not be able to take it off then stick again;
d) The technical-regulation conformity seal may be enlarged or shrunk but the basic proportion and size of the technical-regulation conformity seal must be complied with provision in Annex I of this Circular and may be recognizable with naked eyes;
dd) The technical-regulation conformity seal must be designed and expressed in a same color and recognizable.
Article 5. Methods to assess the conformity
1. The conformity assessment is conducted according to one of the following methods:
a) Method 1: test on typical sample;
b) Method 2: test on typical sample and assess the manufacture process; supervise through testing on sample collected from market;
c) Method 3: test on typical sample and assess the manufacture process; supervise through testing on sample collected from manufacture area in association with assessment of the manufacture process;
d) Method 4: test on typical sample and assess the manufacture process; supervise through testing on sample collected from manufacture area and the market in association with assessment of the manufacture process;
dd) Method 5: test on typical sample and assess the manufacture process; supervise through testing on sample collected from manufacture area or the market in association with assessment of the manufacture process;
e) Method 6: assess and supervise the management system;
g) Method 7: test on and assess a batch of products, goods
h) Method 8: test or verify all products, goods
2. Content, orders and principle of using the methods to assess the conformity are prescribed in the Annex II of this Circular.
Article 6. Applying methods to assess the conformity
1. Methods to assess the standard conformity area applied to each kind of specific product, goods, service, the process, environment which are selected by organizations certifying the standard conformity or organizations, individuals announcing the standard conformity according to the methods to assess the conformity specified in Article 5 of this Circular. The selected method to assess the conformity must be suitable to the assessed object so as to ensure the reliability of result of assessing the conformity.
2.
The methods to assess the technical-regulation conformity applicable to specific products, goods, services, process, and environment are prescribed in the respective technical regulations
3. The methods to assess the conformity must be specified on certificate of the technical-regulation conformity.
ANNEX I
SHAPE AND SIZE OF SEAL OF TECHNICAL-REGULATION CONFORMITY (Enclosed with the Circular No. 28/2012/TT-BKHCN dated December 12, 2012, of the Minister of Science and Technology)
SHAPE AND SIZE OF SEAL OF TECHNICAL-REGULATION CONFORMITY
1. Seal of technical-regulation conformity has the shape as described at picture 1.

Picture 1. Shape of seal of technical-regulation conformity
2. The basic size for designing seal of technical-regulation conformity specified in picture 2.
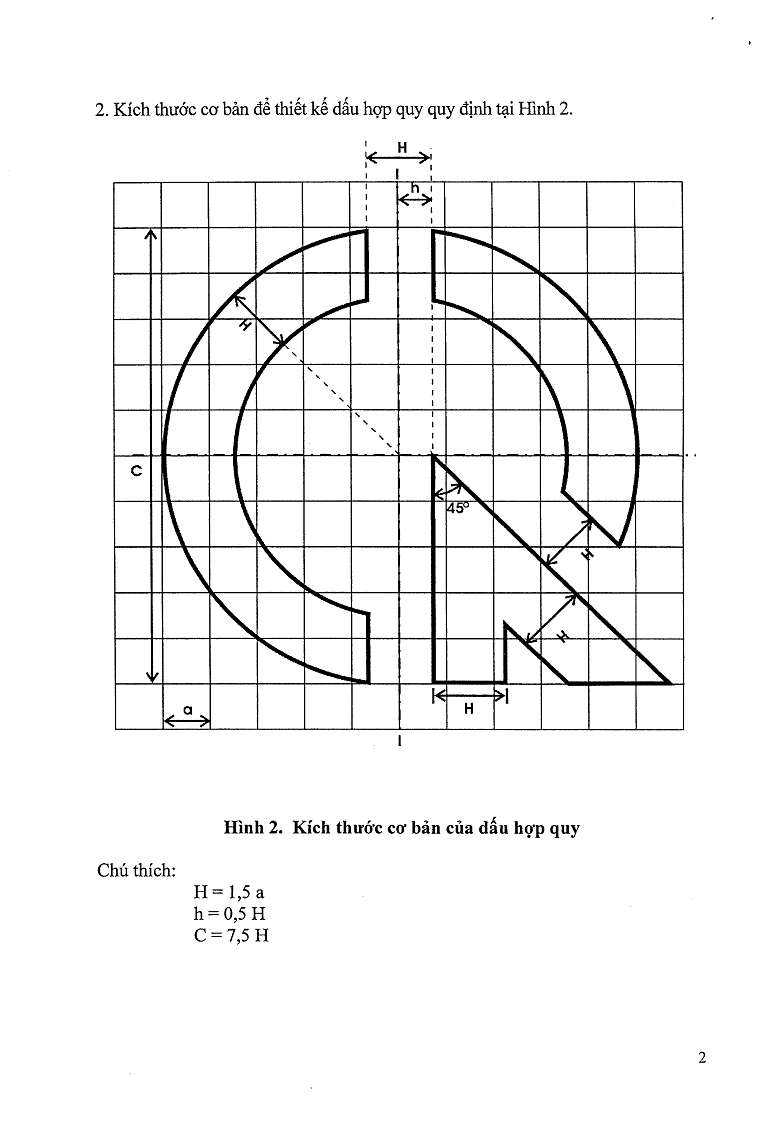
Picture 2. The basic size of seal of technical-regulation conformity
Notes:
H = 1.5 a
h = 0.5 H
C = 7.5 H
ANNEX II
CONTENT, ORDER AND PRINCIPLE OF USING METHODS TO ASSESS THE CONFORMITY (Enclosed with the Circular No. 28/2012/TT-BKHCN dated December 12, 2012, of the Minister of Science and Technology)
CONTENT, ORDERS AND PRINCIPLE OF USING THE METHODS TO ASSESS THE CONFORMITY
I. Method 1: test on typical sample
Method 1 by test on typical sample of products and goods to conclude about the conformity. Conclusion about the conformity is valid for model, type of products and goods which are taken sample for test.
1. Content and order of implementing the main activities in Method 1 include:
Taking sample:
Taking typical samples for products and goods. Typical sample of products and goods is sample representing for a specific model or type of products or goods produced according to a same design, in a same condition and made of same raw materials.
Quantity of samples must be full for testing and storing sample.
1.2. Assessing the conformity of the testing sample:
Samples of products and goods are conducted test at laboratory already registered for operational sector of testing as prescribed by law, may include laboratory of producers. To prioritize for using of laboratory already appointed and recognized.
Characteristics of products, goods for testing and method of testing shall be stipulated in the respective standards and technical regulations.
1.3. Handling of results of assessing the conformity
To consider characteristics of products, goods through result of testing samples in comparison with requirements of the respective standards and technical regulations.
1.4. Conclusion about the conformity
To conclude about the conformity of products and goods in comparison with requirements of the respective standards and technical regulations. Products and goods will be considered as conformable if all criteria of the testing sample are conformable with the prescribed level of the respective standards and technical regulations.
2. Principle of using the method 1
Method 1 is used to assess the conformity of products and goods with the following conditions:
a) Design of products and goods allow defining clearly products and goods according to each typical model, type;
b) Fail to consider requirements to ensure maintenance of quality stability.
II. Method 2: Test on typical samples and assessment on the production process; supervision through testing samples taken on the market
Method 2 bases on result of test on typical samples and assessment of the manufacture process to conclude about the conformity of products and goods. Assessment of supervision is conducted after that and conducted through test on samples of products and goods taken on the market.
1. Content and order of implementing the main activities in Method 2 include:
Taking sample:
Conducting as prescribed in item 1.1 of method 1.
1.2. Assessing the conformity of the testing sample:
Conducting as prescribed in item 1.2 of method 1.
1.3. Assessing the conformity of the production process:
Assessment of production process must consider full conditions of control of producers related to forming products with the aim to ensure maintenance of stability of quality of products and goods. Conditions for control include:
a) Control of technical dossiers of products (design documents, technical standards of products);
b) Control of all production process from input, through intermediate stages, till as finishing products included process of packing, loading and unloading, storage in warehouses and transport of products;
c) Control of quality of raw materials, semi-products and finished products;
d) Control of technological equipment and equipment for metrology, examination and testing;
dd) Control of professional skill qualification of workers and technical officers;
e) Other necessary technical contents.
In case where producers have gotten certificate of quality control system of the certifying organizations registered in sector of certifying or recognized for sector of producing products and goods which are assessed, It is not required to assess the process of production. However, if there is proof about failing to maintain validity of quality control system, the certifying organizations should conduct assessment of the production process, concurrently report to the Directorate for Standards, Metrology and Quality.
1.4. Handling of results of assessing the conformity:
To consider characteristics of products, goods through result of testing samples in comparison with requirements of the respective standards and technical regulations.
To consider the conformity of the production process in comparison with requirements specified in item 1.3 of this method.
1.5. Conclusion about the conformity
To conclude about the conformity of products and goods in comparison with requirements of the respective standards and technical regulations. Products and goods shall be considered as conformable if they ensure full two following conditions:
a) All criteria of the testing sample are conformable to the prescribed level of the respective standards and technical regulations.
b) Result of assessing the production process is conformable to requirement.
Conclusion about the conformity of products and goods will be valid maximally in 3 years, and provided that such products and goods are assessed supervision.
1.6. Supervision:
During valid time of conclusion about the conformity, products and goods must be assessed and supervised through test of samples taken on the market. Frequency of assessment and supervision must ensure not exceeding 12 months once.
The test of samples of products and goods is conducted as prescribed in items 1.1, 1.2 and 1.3 of method 1.
Result of assessing supervision will be used as basis for deciding on maintenance, suspension or cancellation of conclusion about the conformity.
2. Principle of using the method 2
Method 2 is used to assess the conformity of products and goods with the following conditions:
a) Products and goods under subject of having risks involving safety, health and environment at low level.
b) Design of products and goods allow defining clearly products and goods according to each typical model, type;
c) The maintenance of stability and quality characteristics of products and goods should be paid attention during the production;
d) Quality of products and goods able to be changed during course of distribution and circulation on the market;
dd) Organizations and individuals producing and trading in products and goods have effective measures to recall products and goods from the market when detecting unconformable products and goods during supervision.
III. Method 3: test on typical samples and assess the manufacture process; supervise through testing on samples collected from manufacture area in association with assessment of the manufacture process
Method 3 bases on result of test on typical samples and assessment of production process to conclude about the conformity. Assessment of supervision will be conducted through test of samples of products and goods taken from manufacture area in association with assessment of the manufacture process.
1. Content and order of implementing the basic activities in Method 3 include:
Taking sample:
Conducting as prescribed in item 1.1 of method 1.
1.2. Assessing the conformity of the testing sample:
Conducting as prescribed in item 1.2 of method 1.
1.3. Assessing the conformity of the production process:
Conducting as prescribed in item 1.3 of method 2.
1.4. Handling of results of assessing the conformity:
Conducting as prescribed in item 1.4 of method 2.
1.5. Conclusion about the conformity
Conducting as prescribed in item 1.5 of method 2.
1.6. Supervision:
During valid time of conclusion about the conformity, products and goods must be assessed and supervised through test of samples taken at the production area in association with assessment of the production process. Frequency of assessment and supervision must ensure not exceeding 12 months once.
The test of samples of products and goods is conducted as prescribed in items 1.1, 1.2 and 1.3 of method 1.
The assessment of the production process is conducted as prescribed in items 1.3 of method 2.
Result of assessing supervision will be used as basis for deciding on maintenance, suspension or cancellation of conclusion about the conformity.
2. Principle of using the method 3
Method 3 is used to assess the conformity of products and goods with the following conditions:
a) Products and goods under subject of having risks causing loss of safety, health and environment at a level higher than products and goods assessed under the method 2;
b) Design of products and goods allow defining clearly products and goods according to each typical model, type;
c) The maintenance of stability and quality characteristics of products and goods should be paid attention during the production;
d) Quality of products and goods, on nature, is not changed or changed a little during distribution and circulation on the market;
dd) It is difficult to have effective measures to recall products and goods from the market when detecting unconformable products and goods during supervision.
IV. Method 4: test on typical sample and assess the manufacture process; supervise through testing on sample collected from manufacture area and the market in association with assessment of the manufacture process
Method 4 bases on result of test on typical samples and assessment of production process to conclude about the conformity. Assessment of supervision after that will be conducted through test of samples of products and goods taken from manufacture area and market in association with assessment of the manufacture process.
1. Content and order of implementing the basic activities in the Method include:
Taking sample:
Conducting as prescribed in item 1.1 of Method 1.
1.2. Assessing the conformity of the testing samples:
Conducting as prescribed in item 1.2 of Method 1.
1.3. Assessing the conformity of the production process:
Conducting as prescribed in item 1.3 of Method 2.
1.4. Handling of results of assessing the conformity:
Conducting as prescribed in item 1.4 of Method 2.
1.5. Conclusion about the conformity
Conducting as prescribed in item 1.5 of Method 2.
1.6. Supervision:
During valid time of notice about the conformity, products and goods must be assessed and supervised through test of samples taken at the production area and market in association with assessment of the production process. Frequency of assessment and supervision must ensure not exceeding 12 months once.
The test of samples of products and goods is conducted as prescribed in items 1.1, 1.2 and 1.3 of method 1.
The assessment of the production process is conducted as prescribed in items 1.3 of method 2.
Result of assessing supervision will be used as basis for deciding on maintenance, suspension or cancellation of conclusion about the conformity.
2. Principle of using the method 4:
Method 4 is used to assess the conformity of products and goods with the following conditions:
a) Products and goods under subject of having risks causing loss of safety, health and environment at a level higher than products and goods assessed under the method 3;
b) Design of products and goods allow defining clearly products and goods according to each typical model, type;
c) The maintenance of stability and quality characteristics of products and goods should be paid attention during the production;
d) Quality of products and goods is able to lose stability during production, and able to be changed during distribution and circulation on the market;
dd) There are measures allowing recall of products and goods from the market when detecting unconformable products and goods during supervision.
V. Method 5: test on typical sample and assess the manufacture process; supervise through testing on sample collected from manufacture area or the market in association with assessment of the manufacture process.
Method 5 based on result of test on typical samples and assessment of production process to conclude about the conformity. The assessment of supervision is conducted through test on samples taken at production area or on market in association with assessment of the manufacture process.
1. Content and order of implementing the basic activities in Method 5 include:
Taking sample:
Conducting as prescribed in item 1.1 of Method 1.
1.2. Assessing the conformity of the testing samples:
Conducting as prescribed in item 1.2 of Method 1.
1.3. Assessing the conformity of the production process:
Conducting as prescribed in item 1.3 of Method 2.
1.4. Handling of results of assessing the conformity:
Conducting as prescribed in item 1.4 of Method 2.
1.5. Conclusion about the conformity
Conducting as prescribed in item 1.5 of Method 2.
1.6. Supervision:
During valid time of conclusion about the conformity, products and goods must be assessed and supervised through test of samples taken at the production area or on market in association with assessment of the production process. Frequency of assessment and supervision must ensure not exceeding 12 months once.
The test of samples of products and goods is conducted as prescribed in items 1.1, 1.2 and 1.3 of method 1.
The assessment of the production process is conducted as prescribed in items 1.3 of method 2.
Result of assessing supervision will be used as basis for deciding on maintenance, suspension or cancellation of notice about the conformity.
2. Principle of using the method 5:
Method 5 is used to assess the conformity of products and goods with the following conditions:
a) It is necessary to use a method with high reliability as method 4, but it is allowed to be flexible in using supervision measures to cut down costs;
b) It is necessary to use a method which is applied popularly aiming to direct to mutual recognition of results of assessing the conformity.
VI. Method 6: Assessing and supervising the management system
Method 6 bases on assessment of the management system to conclude about the conformity of management system with the respective standards and technical regulations.
1. Content and order of implementing the main activities in Method 6 include:
1.1. Assessing the conformity of management system:
- The management system is assessed under the respective standards and technical regulations.
- Report on result of assessment in comparison with the respective standards and technical regulations.
1.2. Conclusion about the conformity
Base on result of assessment, conclusion about the conformity of management system with the respective standards and technical regulations.
Conclusion about the conformity of management system which is valid maximally 3 years, and provided that management system is assessed and supervised.
1.3. Supervision of management system.
- Supervision through assessment of management system with frequency of assessment and supervision must ensure not exceeding 12 months once.
- Result of assessment is basis for deciding on further maintenance, suspension, cancellation of the conformity of management system.
2. Principle of using the method 6:
Method 6 is used to assess the conformity of process, service, environment with management system under the respective standards and technical regulations.
VII. Method 7: test on and assess a batch of products, goods
The method 7 bases on result of test on samples of products and goods which are taken according to method of statistical probability for a batch of products and goods to give out conclusion about the conformity of batches. Conclusion about the conformity will be valid for the specific batch of products and goods, and it is not required to conduct the following supervision measures.
1. Content and order of implementing the main activities in Method 7 include:
Taking sample:
The test sample is sample taken under method of statistical probability, ensures the representative nature for all batch.
Quantity of samples must be full for testing and storing sample.
1.2. Assessing the conformity of the testing samples:
Conducting as prescribed in item 1.2 of Method 1.
1.3. Handling of results of assessing the conformity:
To consider characteristics of products, goods through result of testing samples in comparison with the respective standards and technical regulations.
1.4. Conclusion about the conformity:
The batch of products and goods will be considered as conformable if quantity of the testing samples with result of non-conformity is in the allowed limitation.
The batch of products and goods will be considered as unconformable with regulations if quantity of the testing samples with result of non-conformity exceeds the allowed limitation.
2. Principle of using the method 7:
The method 7 is used to assess the conformity of products and goods with the following conditions:
a) Products and goods are divided under identical batches;
b) Fail to consider requirements to ensure maintenance of quality stability.
VIII. Method 8: test or verify all products, goods
Method 8 bases on result of testing or verifying all products, goods to conclude the conformity before putting into circulation and use. Conclusion about the conformity will be valid for each single product or goods, and it is not required to conduct the following supervision measures.
1. Content and order of implementing the main activities in Method 8 include:
1.1. Defining the products, goods which need to be tested or verified;
1.2. Assessing the conformity of products, goods:
a) Testing or verifying products and goods is conducted by laboratories, the verifying rooms which have been registered for the capable operational sector at production place, installment place, use place or laboratories, the verifying rooms.
To prioritize for using of laboratories, the verifying rooms already recognized.
b) Characteristics of products, goods which need to be tested, verified, and method of testing, verifying shall be stipulated in the respective standards and technical regulations.
1.3. Handling of results of assessing the conformity:
Consider characteristics of products and goods through result of testing or result of verifying in comparison with requirement.
1.4. Conclusion about the conformity:
Products and goods will be considered as conformable if all criteria of the tested or verified products and goods are conformable with the prescribed level of the respective standards and technical regulations.
2. Principle of using the method 8:
The method 8 is used to assess the conformity of products, goods with strict requirements on safety before putting into circulation and use.
ANNEX III
THE FORMS USED IN ANNOUNCEMENT OF STANDARD CONFORMITY AND ANNOUNCMENT OF TECHNICAL-REGULATION CONFORMITY
(Enclosed with the Circular No. 28/2012/TT-BKHCN dated December 12, 2012, of the Minister of Science and Technology)
1. Plan on quality control:
Form 1. KHKSCL
28/2012/TT-BKHCN.
2. Announcement of standard conformity / announcement of technical-regulation conformity:
Form 2. CBHC/HQ
28/2012/TT-BKHCN.
3. Notice of receiving dossier of announcement of standard conformity / announcement of technical-regulation conformity:
Form 3. TBTNHS
28/2012/TT-BKHCN.
4. Report on situation of receiving dossiers of announcement of standard conformity / announcement of technical-regulation conformity:
Form 4. BCTNHS
28/2012/TT-BKHCN.
5. Report on assessing the standard conformity and technical-regulation conformity:
Form 5. BCDS
28/2012/TT-BKHCN.
Form 1. KHKSCL
28/2012/TT-BKHCN
PLAN ON QUALITY CONTROL
Products/goods/services/process/environment: ……………………………….
|
The specific production processes |
Plan on quality control |
||||||
|
Criteria of supervision and control |
Standards/ technical regulations |
Frequency of taking sample/ size of sample |
Equipment for testing/examination |
Methods of test/examination |
The recording table |
Note |
|
|
(1) |
(2) |
(3) |
(4) |
(5) |
(6) |
(7) |
(8) |
|
|
|
|
|
|
|
|
|
|
|
…………., date........month........year ..…. |
Form 2. CBHC/HQ
28/2012/TT-BKHCN
|
SOCIALIST REPUBLIC OF VIET NAM
ANNOUNCEMENT OF STANDARD CONFORMITY / ANNOUNCEMENT OF TECHNICAL-REGULATION CONFORMITY No. ………………………….. Name of organization or individual: ……… …………………………………………………………………………… Address: ……………………………………………………..……………………………………………… Telephone number: ………………………….. Facsimile: …………………………………………………………… E-mail: ……………………………………………………..……………………………………………… ANNOUNCING: Products, goods, process, services, environment (name, model, type, label, technical characteristic,… ) …………………………..………………………………………..………………………………………… ……………………………………………………………………..……………………………………….. conforming to standard/technical regulations (number, sign, name) …………………………..………………………………………..………………………………………… ……………………………………………………………………..……………………………………….. Added information (based on announcement of standard conformity or announcement of technical-regulation conformity, and method to assess the conformity. ……………………………………………………..………………………………………………………… ……………………………………………………..………………………………………………………… ……………………………………………………..………………………………………………………… .....(Name of organization or individual) …….. hereby commits and takes responsibility for the conformity of………. (products, goods, process, services, environment)… which are produced, traded, preserved, transported, used and exploited by it/him/her.
|
|
|
|
…………., date........month........year …. (Signature, position, seal)
|
Form 3. TBTNHS
28/2012/TT-BKHCN
|
NAME OF SUPERIOR MANAGEMENT AGENCY |
SOCIALIST REPUBLIC OF VIET NAM |
|
No.: …….../TB-…… |
………, date........month........year ….. |
NOTICE
OF RECEIVING DOSSIER OF ANNOUNCEMENT OF STANDARD CONFORMITY / ANNOUNCEMENT OF TECHNICAL-REGULATION CONFORMITY
……. (Name of agency receiving announcement)……….. certifies that dossier of announcing the standard conformity/ the technical-regulation conformity No. ......... dated ........... of ............. (name of organization or individual)…………………
Address of organization or individual: …………………………………………………………………………………
has been received.
For products, goods, process, services, environment (name, model, type, label, technical characteristic,…): ……………………………………………………………………………………………….
conforming to standard (number, sign, name of standard) /technical regulations (number, sign, name of technical regulations) and be valid till date ……………….. (or state: Be valid in 3 years from date ……………..).
This notice records the commitment of organization or individual. This notice has no value to certify that products, goods, services, process, environment are conformable to the respective standards/ technical regulations.
(Name of organization or individual) …………must entirely be responsible for the conformity of products, goods, process, services, and environment, which are produced, traded, preserved, transported, used and exploited by it/him/her.
|
Receivers: |
Competent representative of agency receiving the announcement |
Form 4. BCTNHS
28/2012/TT-BKHCN
|
NAME OF SUPERIOR MANAGEMENT AGENCY |
SOCIALIST REPUBLIC OF VIET NAM |
|
No.: …….../TB-…… |
………, date........month........year ….. |
REPORT
OF RECEIVING DOSSIER OF ANNOUNCEMENT OF STANDARD CONFORMITY / ANNOUNCEMENT OF TECHNICAL-REGULATION CONFORMITY
(From date ….. month …….. year ……… to date ….. month …….. year ………)
|
No. |
The receiving number |
Name of organization or individual conducting announcement |
Name of products, goods, process, services, environment |
Standards/ technical regulations |
Assessment type |
Note |
|
|
The first party (name of the certifying organization already registered / appointed) |
The third party (self assessment) |
|
|||||
|
1 |
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
.... |
|
|
|
|
|
|
|
Total dossiers of announcement of standard conformity / announcement of technical-regulation conformity already received:…………………………………………………………
|
Receivers: |
Competent representative of agency receiving the announcement |
Form 5. BCDG
28/2012/TT-BKHCN
|
NAME OF SUPERIOR MANAGEMENT AGENCY (if any) |
SOCIALIST REPUBLIC OF VIET NAM |
|
No.: ……...…… |
………, date........month........year ….. |
REPORT
ON ASSESSING THE STANDARD CONFORMITY AND TECHNICAL-REGULATION CONFORMITY
1. Assessment date: ..............................................................................................................
2. Assessment place: ..............................................................................................................
3. Product name: ..............................................................................................................
4. Number of standards / technical regulations applied: .............................................................................
5. Name of organization testing the product: ................................................................
6. Assessment on result of testing according to the applied standards / technical regulations and the effectiveness of application, implementation of the production process: ............................................................................. .................................... ............................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
7. Other content (if any): .................................... .................................... .............................
8. Conclusion:
Product conforms to standard/technical regulations.
Product fails to conform to standard/technical regulations.
|
The assessing person (signature and full name) |
Certified by leader of organization or individual |
|
A Quick Intro |
Search Trade Information
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Feature Information
|
|
|
|
|
|
|
|
|
|
|
Information & Articles
|
|
|
|
|
|
|
|
|
|
|
|
Contact Us! If you cannot find what you require in this website please feel free to contact us. Click here to send us a message >>>
|