View Procedure
| Procedure Name | Granting permission to import vaccines and medical biological products being serum used for disease prevention and treatment under national target health programs |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address:
Phone:
Email:
|
|
Legal base of the Procedure
|
- Law on Pharmacy No. 105/2016/QH13
- Decree 102/2016/NĐ-CP on drug business requirements
- Decision 151/2007/QD-TTg dated September 12, 2007, promulgating the Regulation on the Import of Drugs without Registration Numbers in Vietnam
- Circular 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export, import of drugs/medicines and packaging in direct contact with drugs/medicines
|
|
Processing time
|
- Within 15 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
VND 0
|
Required documents:
|
No
|
Type of documents
|
Note
|
|
1
|
Import order (Form 9);
|
01 original copy
|
|
2
|
Documents issued by the competent authorities on the import of medicines for the national target health program
|
01 original copy
|
|
3
|
A certificate of the competent authority of the home country for permitting the circulation or export of vaccines and medical biological products;
|
01 original copy
|
|
4
|
Certificate of production establishment obtaining GMP standard;
|
01 original copy
|
|
5
|
Testing paper of obtaining the quality standard of vaccines, medical bio-products of the national accreditation agency or other competent authorities of the home country for lots of imported goods
|
01 original copy
|
|
6
|
Clinical test results on or safety test results on human body
|
01 original copy
|
Conditions:
|
Vaccines, medical biological products without registration numbers, when imported into Vietnam they must have a remaining expiry date of at least by 2/3 of expiry date from the date of arrival in Vietnam
Vaccines, medical biological products with valid circulation registration numbers in Vietnam, when imported into Vietnam, they must have a remaining expiry date of at least by 1/2 of expiry date from the date of arrival in Vietnam
Biological products used in In Vitro diagnosis with expiry date of equal to or less than 12 months when imported into Vietnam, they must have a remaining expiry date of at least by 3 months from the date of arrival in Vietnam
Drugs/medicines of the national health target program must be entrusted through the enterprises with direct importing function of medicines. On the drug/medicine’s label must bear the phase "program medicine, not for sale".
|
Process Steps
|
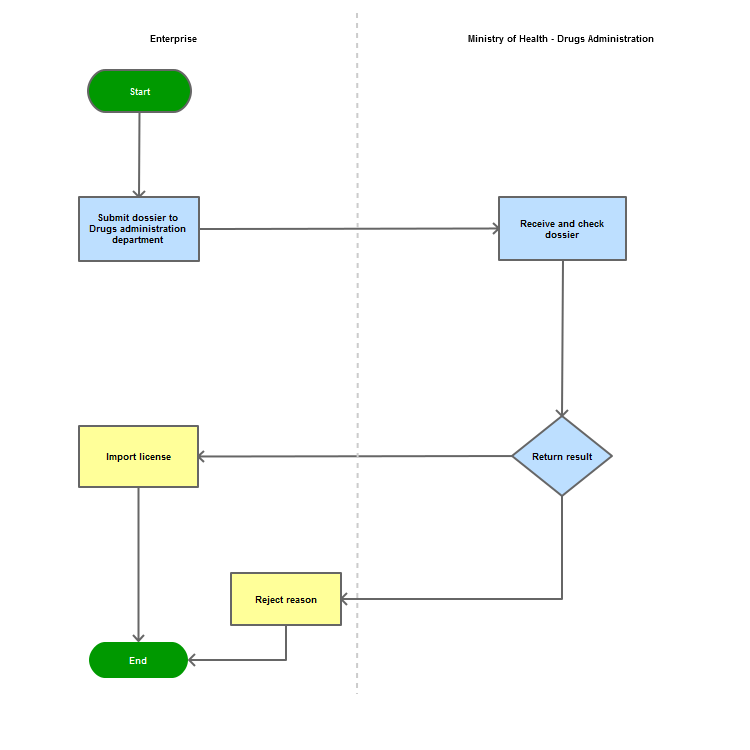
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
The Administration shall inform the applicant of the results.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map:
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures