View Procedure
| Procedure Name | Granting permission to import drugs/medicines for clinical trials |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address:
Phone:
Email:
|
|
Legal base of the Procedure
|
- Law on Pharmacy No. 105/2016/QH13
- Decision 151/2007/QD-TTg dated September 12, 2007, promulgating the Regulation on the Import of Drugs without Registration Numbers in Vietnam
- Circular 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export, import of drugs/medicines and packaging in direct contact with drugs/medicines
|
|
Processing time
|
- Within 15 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
VND 0
|
Required documents:
|
No
|
Type of documents
|
Note
|
|
1
|
Import order (Forms 11a, 11b, 11c)
|
01 original copy
|
|
2
|
The research plan of clinical trials approved by the Minister of Health
|
01 original copy
|
Process Steps
|
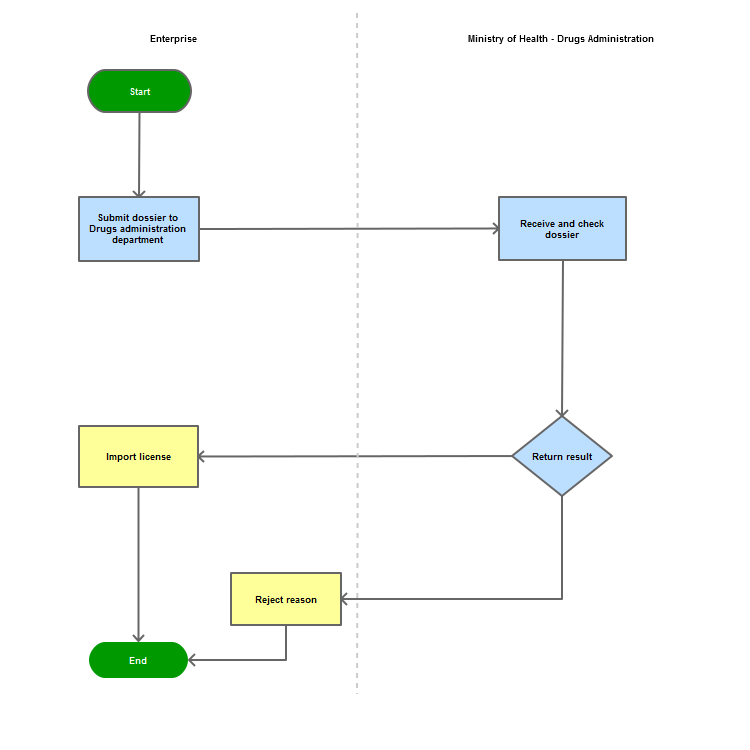
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
The Administration shall inform the applicant of the results.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map:
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures