View Procedure
| Procedure Name | Granting permission to import raw materials for addictive drugs/medicines | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
Required documents:
Conditions:
Process Steps
Process Map:
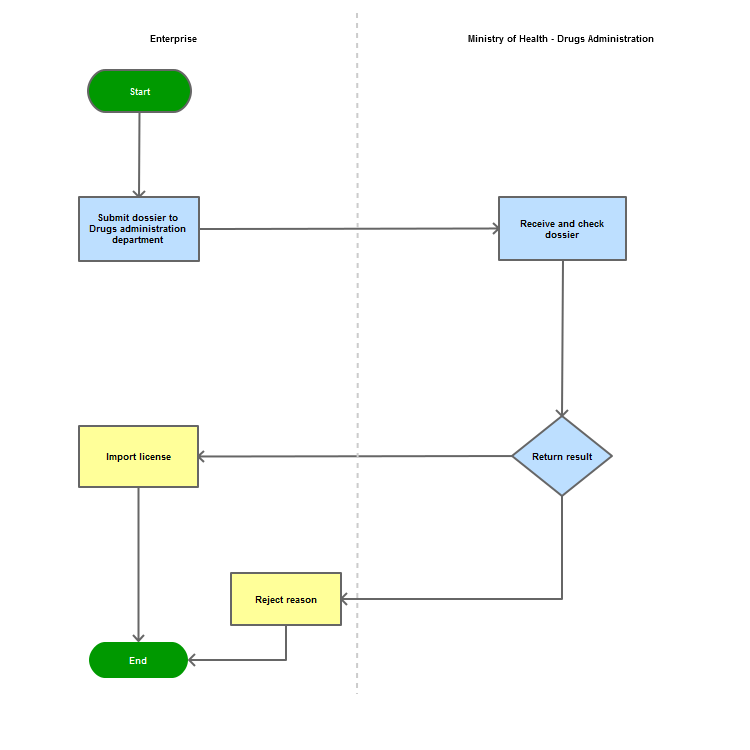 | |||||||||||||||||||||||||||||
| Category | Procedure |
The following form/s are used in this procedure
| Title | Description | Created Date | Updated Date | Issued By |  |
|---|---|---|---|---|---|
| Form 4 - Addictive inventory report | Form 4 - Addictive inventory report | 28-05-2016 | 09-06-2016 | Ministry of Health | |
| Form 2d List of imported addictive drugs material | Form 2d List of imported addictive drugs material | 07-06-2016 | 09-06-2016 | Ministry of Health |
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| Importing addictive materials and precursor substances | Permit Requirement | Ministry of Health | Importing addictive materials and precursor substances | Importing addictive materials and precursor substances | Circular 47/2010/TT-BYT Guiding the export, import of medicines and packaging directly contacted with medicines | 31-12-9999 | Good |
