View Procedure
| Procedure Name | Granting permission to import medicine materials and pharmaceuticals without registration numbers |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address:
Phone:
Email:
|
|
Legal base of the Procedure
|
- Law on Pharmacy No. 105/2016/QH13
- Decision 151/2007/QD-TTg dated September 12, 2007, promulgating the Regulation on the Import of Drugs without Registration Numbers in Vietnam
- Circular 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export, import of drugs/medicines and packaging in direct contact with drugs/medicines
|
|
Processing time
|
- Within 15 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
|
Required documents:
|
No
|
Type of documents
|
Note
|
|
1
|
Import order (Form 12a, 12b, 12c)
|
01 original copy
|
|
2
|
Quality standards and testing methods of materials, pharmaceutical materials, packaging in direct contact with the drugs/medicines for the raw materials, packaging with quality standards and testing methods of the manufacturers or copy of quality standards treatise of pharmacopoeia if raw materials are not applied the quality standards of the Pharmacopeia of Europe, the United Kingdom, United States, International Pharmacopeia, and of Japan
|
01 original copy
|
Requirements and conditions in following administrative procedures:
|
1
|
Conditions and scope of organizations and individuals participating in export and import of drugs/medicines, packaging in direct contact with drugs/medicines
- Enterprises having certificates of eligibility for drug/medicine trading and drug/medicine warehouses obtaining standard "Good Storage Practice" (GSP) are imported directly and received entrusting to import finished drugs, addictive ingredients, psychotropic substances and precursor substances with registration numbers for circulation in accordance with the business scope stated in the certificate of eligibility for drug/medicine trading and GSP certificate;
Enterprises having certificates of eligibility for drug/medicine trading and certificate obtaining standards "Good Manufacturing Practice" (GMP) are imported raw materials for medicine manufacture of such enterprises and sold to other medicine production enterprise;
Production enterprises having certificates of eligibility for drug/medicine trading from medicinal materials are imported to serve the needs of enterprises’ manufacturing and sold to other medicine production establishments, the traditional examination and treatment establishments;
|
|
2
|
For traders who are enterprises with direct foreign-owned capital in Vietnam having certificates of eligibility for drug/medicine trading (the scope of medicine production) are imported raw materials for medicine production of such enterprises. The export and import of drugs/medicines not serving for medicine production of such enterprises will be guided by the Ministry of Health in other documents.
|
|
3
|
The Ministry of Health shall grant the import permit if one of the following conditions are met:
Raw materials for making medicines imported into Vietnam, except for pharmaceutical materials, must have remaining expiry date of
more than 36 months from the date of arrival in Vietnam, for raw materials with expiry date of equal to or less than 36 months, the arrival date of the Vietnam's ports is not exceeded 06 months from date of manufacture;
|
Process Steps
|
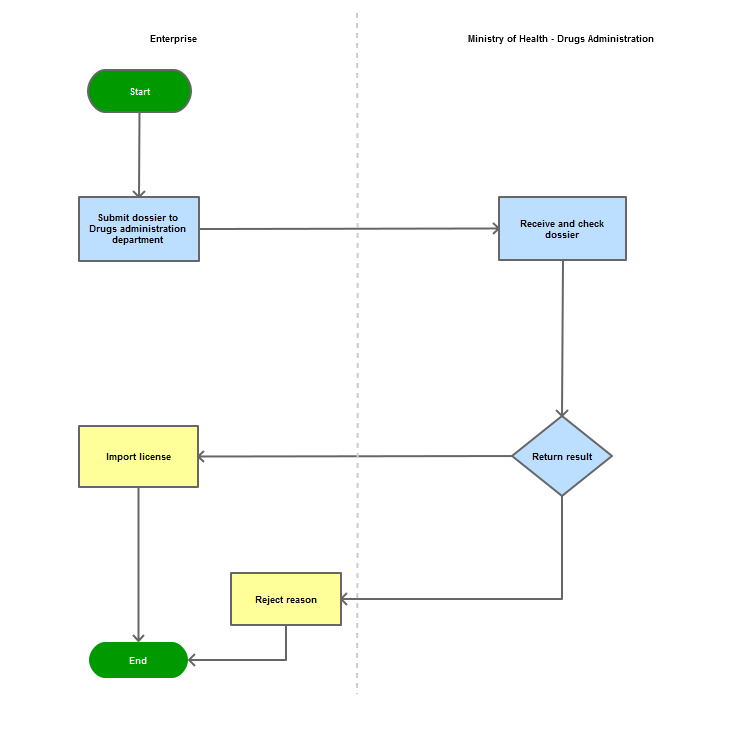
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
The Administration shall inform the applicant of the results.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map:
|
|---|
| Category | Procedure |
|---|
Please customize this view
This procedure apply to theses Measures.