View Procedure
| Procedure Name | Granting permission to export finished addictive drugs, psychotropic substances, precursor substances with/without registration numbers |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address:
Phone:
Email:
|
|
Legal base of the Procedure
|
-
Law on Pharmacy No. 34/2005/QH11
-
Decree No. 79/2006/ND-CP
-
Circular No. 10/2010/TT-BYT dated April 29, 2010 guiding activities related to addictive drugs
-
Circular No. 11/2010/TT-BYT dated April 29, 2010 of the Ministry of Health guiding activities related to psychotropic substances and precursor substances
-
Circular No. 45/2011/TT-BYT amending and supplementing a number of articles in documents regulating pharmaceutical and cosmetic industries.
-
Circular No. 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export, import of drugs/medicines and packaging in direct contact with drugs/medicines
|
|
Processing time
|
-
Within 15 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
VND 0
|
Required documents:
|
No
|
Type of documents
|
Note
|
|
1
|
Export order (Form 13a)
|
01 original copy
|
|
2
|
Written permission for import of the competent authority of the importing country;
|
01 original copy
|
Requirements and conditions in following administrative procedures:
|
Central Pharmaceutical Company 1, Central Pharmaceutical Company 2, Central Pharmaceutical Company 3, SAFACO, YTECO, HAPHARCO. are entities entitled to export raw materials for addictive drugs, psychotropic substances and precursor substances
|
Process Steps
|
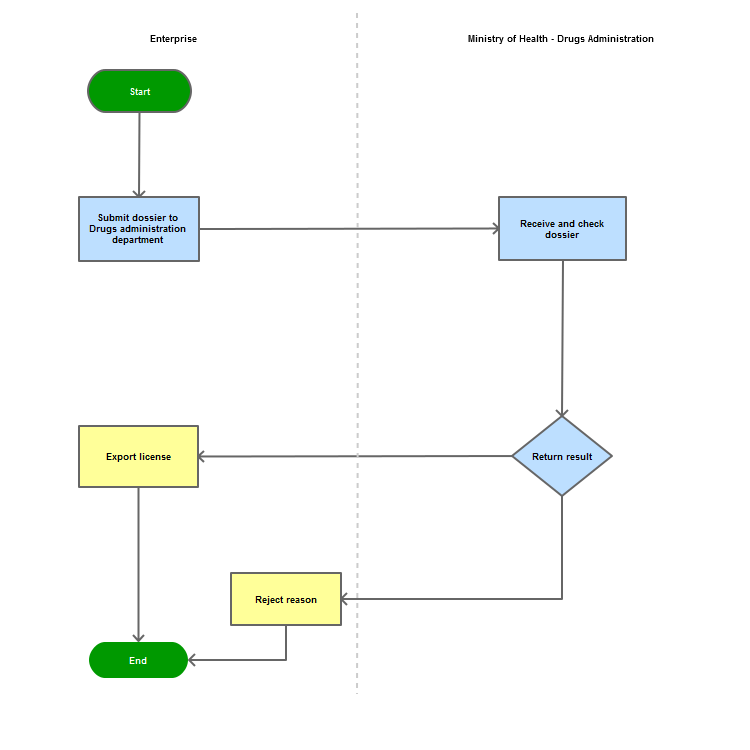
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
The Administration shall inform the applicant of the results.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map:
|
|---|
| Category | Procedure |
|---|
Please customize this view
This procedure apply to theses Measures.