| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Ministry of Health - Department of Medical Equipment and Health Facilities
Address:
Phone:
Email:
|
|
Legal base of the Procedure
|
- Law on Product and Goods Quality No. 05/2007/QH12 dated November 21, 2007.
- Commercial Law No. 36/2005/QH11
- Decree 187/2013/ND-CP dated November 20, 2013 of the Government, detailing the Commercial Law regarding international goods purchase and sale and goods purchase and sale agency, processing and transit activities with foreign partners
- Decree 43/2017/ND-CP on goods labeling applied to goods in circulation in Vietnam and goods for export/import
- Circular 278/2016/TT-BTC dated November 14, 2016, providing for fees in medical sector, and the collection, transfer, management and usage
- Circular 30/2015/TT-BYT on import of medical tools and equipment
|
|
Processing time
|
- Within 15 working days from the date of reception of the adequate and valid dossier
|
|
Fee
|
Medical tools & equipment
VND 200,000/Item
Imported medical equipment priced at below 1 billion VND
VND 500,000/Item
Imported medical equipment priced 1 - 3 billion VND
VND 1,000,000/Item
Imported medical equipment priced at over 3 billion VND
VND 3,000,000/Item
|
Required documents:
|
No
|
Type of documents
|
Note
|
|
1
|
Application for import permit certified and sealed by person with legal responsibility or person of authorization (Annex 2)
|
01 original copy
|
|
2
|
The valid ISO 13485 or ISO 9001 quality systems certification (ISO Certification) of the manufacturer/country of origin applied to imported medical equipment
|
01 original copy
|
|
3
|
The valid Certificate of Free Sale (CFS) applied to imported medical equipment or FDA Certificate of Free Sale or CE Mark Certificate (original copy or photocopy certified in Vietnam or consularly legalized by the Vietnamese Diplomatic Mission or Vietnam Embassy in the country of origin)
|
01 original copy
|
|
4
|
The valid written authorization of the manufacturer or eligible distributor permitting the importing entity to import medical equipment into Vietnam (original copy or photocopy certified in Vietnam or consularly legalized by the Vietnamese Diplomatic Mission or Vietnam Embassy in the country of origin)
|
01 original copy
|
|
5
|
01 photocopy appended with the registrant's seal of the Import Permit already granted by the Ministry of Health earlier
|
01 original copy
|
Requirements and conditions in following administrative procedures:
|
1
|
Legal conditions:
Certificate of business registration or Certificate of investment containing the registered line of business of importing medical tools and equipment
|
|
2
|
Personnel conditions:
a) The person in charge of technical matters must satisfy one of the following requirements: University diploma in biomedical electronics, biomedical physics or university diploma in such majors as engineering, medicine, pharmacy and have specialized training certificate in medical equipment granted by institutions eligible in training technical engineering of medical tools and equipment or equivalent certificate granted by a foreign institution with a field of training suitable to the medical tools and equipment under request for import.
For persons holding university diploma in such majors as engineering, medicine, pharmacy and with experience of direct working on engineering of medical tools and equipment or holding positions relating to the management of medical equipment in eligible medical facilities in at least 3 years as certified by the heads of their working units, specialized training certificates in medical equipment are not required.
b) Having qualified officers and technical staff in charge of guiding the installation, warranty, maintenance of medical tools/equipment related to the imported equipment.
|
|
3
|
Infrastructure conditions:
a) Having facilities and warehouses to properly maintain medical equipment, ensuring medical equipment to be stored with proper conditions, to be protected from: light, temperature, humidity and other conditions.
b) Having means of prevention and fighting of fire and explosion and ensuring safety, hygiene, and the environment as specified by laws
|
|
4
|
Labeling of imported medical equipment: follow the provisions of Decree No. 43/2017/ND-CP on goods labeling and Circular No. 09/2007/TT-BKHCN of Ministry of Science and Technology guiding the implementation of a number of articles of Decree 89/2006/ND-CP by the Government on goods labeling and other related regulations
|
Process Steps
|
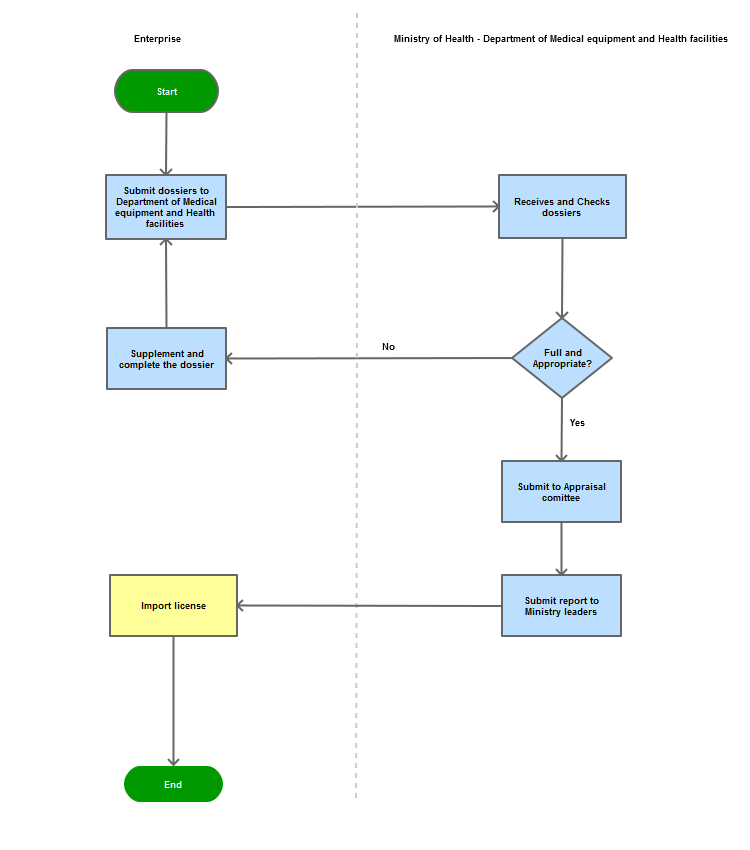
Step 1
|
The applicant shall submit a dossier of application for import of medical tools and equipment to the Department of Medical Equipment and Health Facilities
|
|
Step 2
|
The Department shall receive and examine the dossier. Issue a request for correction or submission of supplementary document(s) if the dossier is not sufficient or valid. If the dossier is sufficient and valid, the Department shall examine and make a Record of assessment to submit to the leadership of the Ministry.
|
|
Step 3
|
Grant the import permit to the applicant.
|
Process Map:
|
|---|