View Procedure
| Procedure Name | Applying for announcement of conformity for imported products (supplements and nutrient foods not included) aiming to be used internally in production facilities, supermarkets, hotels of four stars or higher rank |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Ministry of Health – Vietnam Food Administration
Address: 138A Giang Vo – Ba Dinh - Hanoi
Phone: 04.38464489.04.38463702
Email: vfa@vfa.gov.vn
|
|
Legal base of the Procedure
|
- Decree 38/2012/ND-CP detailing the implementation of a number of articles of the Law on Food Safety
- Circular 19/2012/TT-BYT guiding the announcement of conformity or announcement of compliance with food safety regulations.
|
|
Processing time
|
Within 15 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
150,000 VND/Item
|
Required Documents
|
No.
|
Document Title
|
Note
|
|
Dossier of announcement on conformity with regulations on food safety (2 sets)
|
|
1
|
The product declaration
- For food raw materials, packing materials, instruments in direct contact with import food just aiming to service for internal production of enterprises: The declaration specified in Form 4 enclosed with Circular 19/2012/TT-BYT.
- For imported food additives, food processing enhancers just aiming to service internal production of enterprises: The declaration specified in Form 2 enclosed with Circular 19/2012/TT-BYT.
- For imported food just aiming to trade in supermarkets, hotels of four stars or higher rank: The declaration specified in Form 3 enclosed with Circular 19/2012/TT-BYT.
|
01 original copy
|
|
2
|
The results of testing product within 12 months of producers or the details on product information of producers; or results of testing product of the appointed testing laboratory or recognized independent testing laboratory or accepted testing laboratory.
|
01 original copy
|
|
General legal documents (1 set)
|
|
1
|
A business registration certificate with the food business line or legal entity certificate for organization and individual importing food (copy certified by the organization or individual);
|
01 certified photocopy
|
Process Steps
|
Step 1
|
The organization/individual shall submit the dossier to the Vietnam Food Administration - Ministry of Health
|
|
Step 2
|
The Vietnam Food Administration shall receive and examine the dossier:
|
|
Step 3
|
Reply with the written certification of the announcement of conformity with food safety regulations. In case of disapproval, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map: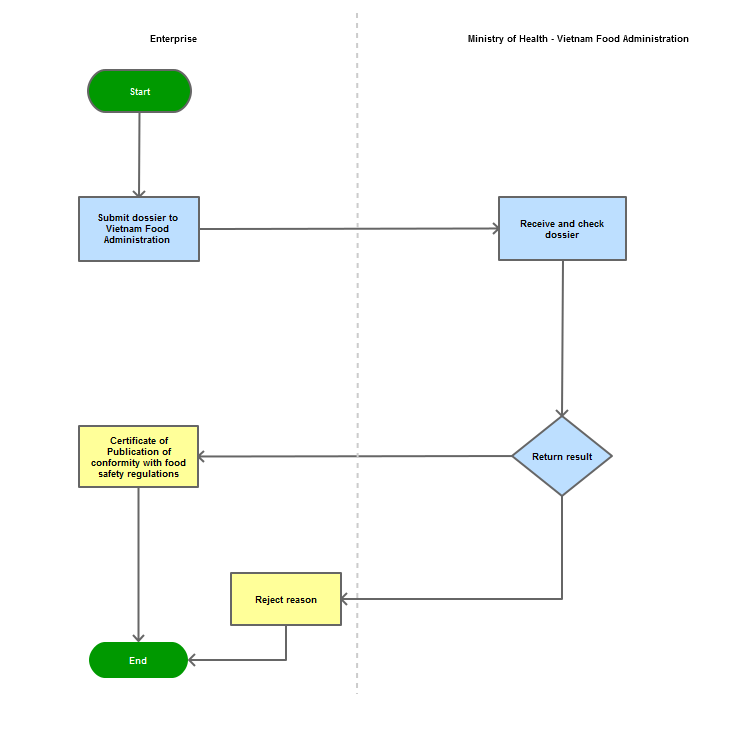
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures


