View Procedure
| Procedure Name | Granting permission for organizations/individuals to export/import finished drugs/medicines that contain an amount of addictive substances and for organizations to export/import drugs under non-commercial regime |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address: 138A Giang Vo - Ba Dinh - Hanoi
Phone: (+844) 3736 (ext:
Email: cqldvn@moh.gov.vn
|
|
Legal base of the Procedure
|
- Circular 39/2013/TT-BYT prescribing the management of curative drugs/medicines for human use imported/exported under non-commercial regime
- Circular 05/2016/TT-BYT regulating medications prescribed for outpatients
|
|
Processing time
|
Within 07 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
VND 0
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
An application form (Form 1a/PMD or Form 1b/PMD)
|
01 original copy
|
|
2
|
A copy of the identity card or passport of the person importing or exporting the drugs/medicines under non-commercial regime (to produce the original of the identity card or passport when carrying out customs procedures)
|
01 photocopy
|
|
3
|
Attached documents: A prescription or medical examination book or medical records or related documents
|
01 original copy
|
|
4
|
A prescription made up by a Vietnamese doctor or an outpatient medical examination book made according to the form specified in Appendix I of Circular No. Circular No. 05/2016/TT-BYT regulating medications prescribed for outpatients. A prescription made up by a foreign doctor or an outpatient medical examination book made according to the contents specified in Clause 1 Article 5 off Circular No. 05/2016/TT-BYT regulating medications prescribed for outpatients.
|
01 original copy
|
Process Steps
|
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
Reply the applicant with results within 7 days.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map: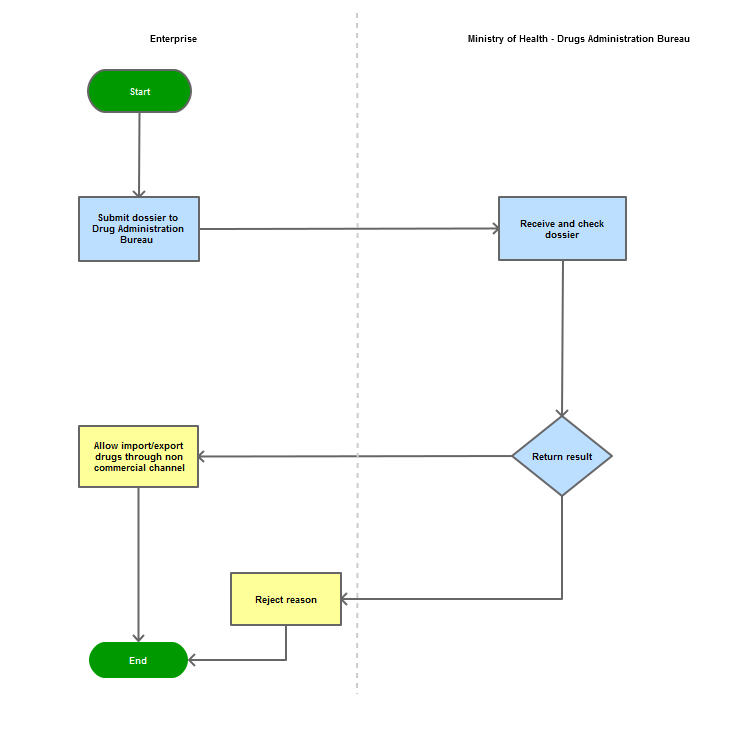
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Non-commercial imported or exported addictive medicines | Permit Requirement | Ministry of Health | 1. Non-commercial imported or exported medicines shall be used only for treatment of diseases of individuals who request the non-commercial import or export of medicine and individuals working in organizations requesting the non-commercial import or export of medicines or their family members. Non-commercial imported medicines may neither be sold in the market nor used for any other illegal purposes.
2. Individuals and organizations requesting the non-commercial import or export of medicines shall take responsibility for the origin and quality of such medicines.
3. Non-commercial imported or exported medicines must have labels indicating their appellations, active ingredients, concentrations, contents and expiry date. | Medicines containing active ingredients on the list of active ingredients banned from import or export for use as medicines prescribed in the Minister of Health’s Circular No. 47/2010/TT-BYT of December 21, 2010, guiding medicine import and export, documents amending and supplementing this Circular, and official letters of the Ministry of Health on the suspension of medicine use and import | Circular 39/2013/TT-BYT Prescribing the management of curative medicines for human use which are imported and exported through the non commercial channel. | 31-12-9999 | Good |
237


