View Procedure
| Procedure Name | Granting permission for organizations/individuals to import/export finished drugs/medicines not containing addictive substances under non-commercial regime under the management of the Provincial Health Department |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Provincial Health Department
Address: 138A Giang Vo – Ba Dinh - Hanoi
Phone: (+844) 3736 (ext:
Email: cqldvn@moh.gov.vn
|
|
Legal base of the Procedure
|
- Circular 39/2013/TT-BYT prescribing the management of curative drugs/medicines for human use imported/exported under non-commercial regime
- Circular 05/2016/TT-BYT regulating medications prescribed for outpatients
|
|
Processing time
|
- Within 07 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
An application form (Form 1a/PMD or Form 1b/PMD)
|
01 original copy
|
|
2
|
A copy of the identity card or passport of the person importing or exporting the drugs/medicines under non-commercial regime (to produce the original of the identity card or passport when carrying out customs procedures)
|
01 photocopy
|
|
3
|
Attached documents: A prescription or medical examination book or medical records or related documents
|
01 original copy
|
|
4
|
A prescription made up by a Vietnamese doctor or an outpatient medical examination book made according to the form specified in Appendix I of Circular No. Circular No. 05/2016/TT-BYT regulating medications prescribed for outpatients. A prescription made up by a foreign doctor or an outpatient medical examination book made according to the contents specified in Clause 1 Article 5 off Circular No. 05/2016/TT-BYT regulating medications prescribed for outpatients.
|
01 original copy
|
Process Steps
|
Step 1
|
The applicant shall submit the dossier to the Provincial Health Department
|
|
Step 2
|
The Department shall receive and examine the dossier.
|
|
Step 3
|
Reply the applicant with results within 7 days.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map: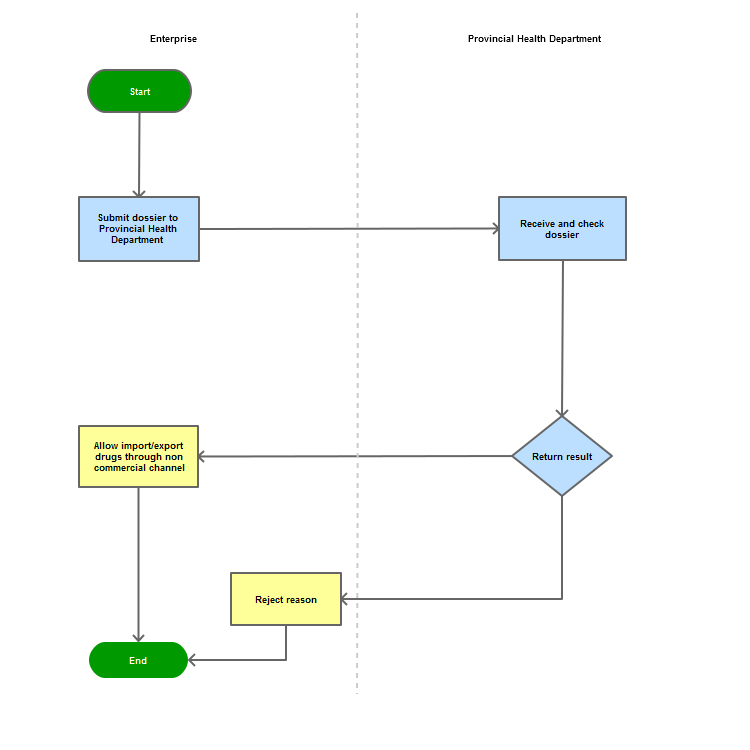
|
|---|
| Category | Procedure |
|---|
Please customize this view
This procedure apply to theses Measures.

