View Procedure
| Procedure Name | Procedure for getting license for export addictive drugs, psychotropic, combined substances for circulation registration, exhibition, research |
|---|
| Description |
| Category |
License/Permit |
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address: 183A Giang Vo – Ba Dinh - Hanoi
Phone: +844 3736 6483
Email: cqldvn@moh.gov.vn
|
| Legal base of the Procedure |
- Law on Pharmacy No. 105/2016/QH13
- Decree 102/2016/ND-CP on drug business requirements
- Circular 19/2014/TT-BYT Management of addictive drugs, psychotropic drugs, and drug precursors
- Circular 45/2011/TT-BYT amending and supplementing a number of articles in documents regulating pharmaceutical and cosmetic industries.
- Circular 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export, import of drugs/medicines and packaging in direct contact with drugs/medicines
|
| Processing time |
- Within 15 working days from the date of reception of adequate and valid dossier
|
| Fee |
|
Required Documents
|
No.
|
Document Title |
Note |
|
1
|
Export order (Form 13a) |
01 original copy |
|
2
|
Written explanation of the exporters specifying reasons and purposes of the export |
01 original copy |
Requirements and conditions:
| Enterprises having certificates of eligibility for drug/medicine trading and drug/medicine warehouses obtaining standard "Good Storage Practice" (GSP) are eligible to import directly and receive entrusting to import finished drugs containing addictive substances in combined forms, psychotropic substances and precursor substances with registration numbers for circulation |
Process Steps
|
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health |
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier. |
|
Step 3
|
The Administration shall inform the applicant of the results.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map: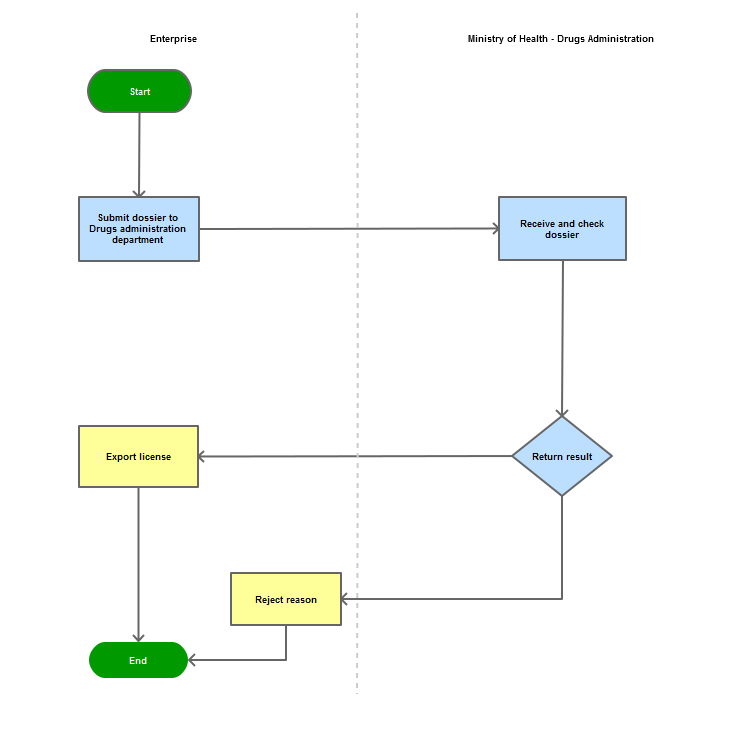
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Export of Addictive medicines, psychotropic drugs, pre-substances used as medicines | Licensing Requirement | Ministry of Health | Addictive medicines, psychotropic drugs, pre-substances used as medicines, including those in single or mixed substances when exporting, it is required to have export permit of the Drugs Administration Department- Ministry of Health | Medicines, packaging in direct contact with medicines are not addictives as exporting, exporters make procedures directly with the customs of border gate, without required to have export permit of the Ministry of Health. | Circular 47/2010/TT-BYT Guiding the export, import of medicines and packaging directly contacted with medicines | 31-12-9999 | Good |
239


