View Procedure
| Procedure Name | Granting permission to import rare drugs/medicines for treatment needs of specific hospitals in special cases |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address: 183A Giang Vo – Ba Dinh - Hanoi
Phone: +844 3736 6483
Email: cqldvn@moh.gov.vn
|
|
Legal base of the Procedure
|
- Law on Pharmacy 105/2016/QH13
- Decree 102/2016/ND-CP on Drug business requirements
- Decision 37/2008/QD-BYT issuing the list of rare drugs/medicines
- Circular 45/2011/TT-BYT amending and supplementing a number of articles in documents regulating pharmaceutical and cosmetic industries.
- Circular 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export, import of drugs/medicines and packaging in direct contact with drugs/medicines
|
|
Process time
|
- Within 7 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
Import order (Form 6a)
|
01 original copy
|
|
2
|
Reserves for rare medicines, medicines for the treatment needs of the hospital in special cases (Form 6b)
|
01 original copy
|
|
3
|
Certificate of Free Sale (FSC)
|
01 original copy
|
|
4
|
"Good Manufacturing Practice" (GMP) certificate or CPP
|
|
|
5
|
Standards and testing methods
|
01 original copy
|
|
6
|
01 set of original labels enclosed with the user guides in actual circulation in the country of origin
|
01 set
|
|
7
|
02 sets of labels intended to be put in circulation in Vietnam enclosed with user guides already translated into Vietnamese.
|
02 sets
|
Requirements and conditions:
|
1
|
Dare drugs, specific drugs or drugs of special preparation forms to meet treatment needs
|
|
2
|
Enterprises importing drugs/medicines on the list of rare drugs/medicines, drugs/medicines imported for the needs of treatment of a specific hospital in special cases or the drugs/medicines with active substances, concentrations, contents, dosage forms without registration numbers for circulation in Vietnam but recorded in specialized documents but unable to offer the specified records and original testing sheet of the imported drug/medicine lots, are exempt from submitting documents as specified in Clauses 3, 4, 5, 6, 7. However, they are required to supplement a written statement of reasons for delay in providing records of imported medicines, original testing sheet of the drug/medicine lot and commitment on ensuring the quality of imported drugs/medicines; report of drug/medicine use (demand for use, safety and treatment effectiveness of the drugs/ medicines).
|
|
3
|
- Enterprises having certificates of eligibility for drug/medicine trading and drug/medicine warehouses obtaining standard "Good Storage Practice" (GSP) are eligible to import directly and receive entrusting to import rare drugs/medicines for the treatment needs of specific hospitals in special cases in accordance with the business scope stated in the Certificate of eligibility for drug/medicine trading and GSP certificate;
|
Process Steps
|
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
The Administration shall inform the applicant of the results.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map: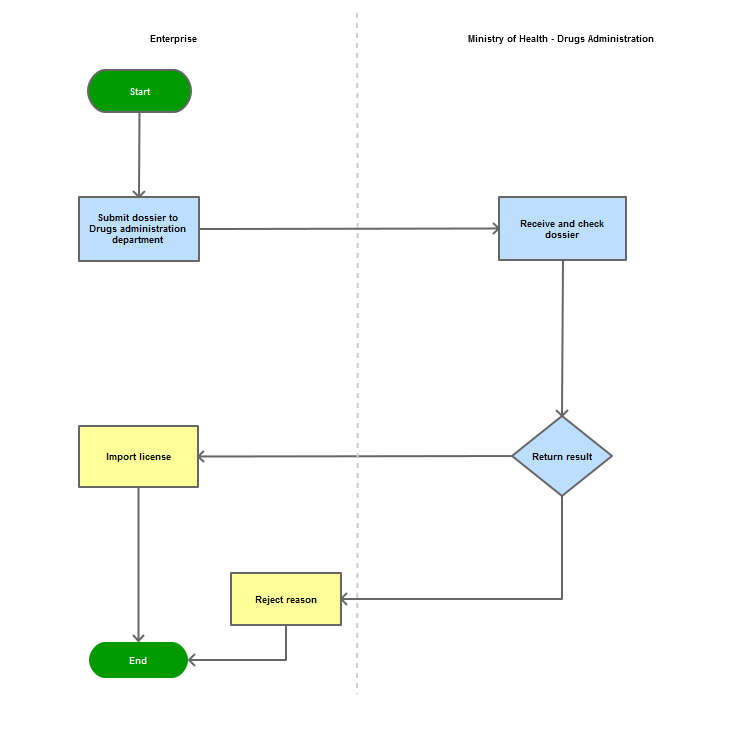
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures


