View Procedure
| Procedure Name | Granting permission to the import order of rare drugs/medicines to be distributed to chains of pharmacies meeting the standard GPP |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address: 183A Giang Vo – Ba Dinh - Hanoi
Phone: +844 3736 6483
Email: cqldvn@moh.gov.vn
|
|
Legal base of the Procedure
|
- Law on Pharmacy No. 105/2016/QH13
- Decree 102/2016/ND-CP on Drug business requirements
- Decision 37/2008/QD-BYT issuing the list of rare drugs/medicines
- Circular 45/2011/TT-BYT amending and supplementing a number of articles in documents regulating pharmaceutical and cosmetic industries.
- Circular 47/2010/TT-BYT dated December 29, 2010 of the Ministry of Health guiding the export, import of drugs/medicines and packaging in direct contact with drugs/medicines
|
|
Process time
|
- Within 7 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
Import order (Form 6a)
|
03 original copy
|
|
2
|
Written statement of reasons for delay in providing records of imported medicines, original testing sheet of the drug/medicine lot and commitment on ensuring the quality of imported drugs/medicines (in case of failure to provide these materials).
|
01 original copy
|
Requirements and conditions:
|
1
|
Dare drugs, specific drugs or drugs of special preparation forms to meet treatment needs
|
|
2
|
Enterprises having certificates of eligibility for drug/medicine trading and drug/medicine warehouses obtaining the standard "Good Storage Practice" (GSP) are eligible to import directly and receive entrusting to import rare drugs/medicines for the treatment needs of specific hospitals in special cases in accordance with the business scope stated in the Certificate of eligibility for drug/medicine trading and GSP certificate
|
|
3
|
Enterprises organizing chains of pharmacies obtaining the standard "good pharmacy practice" GPP in need of importing drugs/medicines in the list of rare drugs and medicines imported for the treatment needs of specific hospitals in special cases to sell at the pharmacies obtaining GPP in the system: They must submit a written statement of reasons for delay in providing records of imported medicines, original testing sheet of the drug/medicine lot and commitment on ensuring the quality of imported drugs/medicines.
|
|
4
|
Enterprises having already received the written announcement of "The Enterprise organizing a chain of pharmacies obtaining the standard "good pharmacy practice" GPP" certified by the Drug Administration of Vietnam
|
Process Steps
|
Step 1
|
The applicant shall submit a dossier of Application to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier.
|
|
Step 3
|
The Administration shall inform the applicant of the results.
In case of refusal, the Administration shall reply in writing and clearly state relevant reasons.
|
Process Map: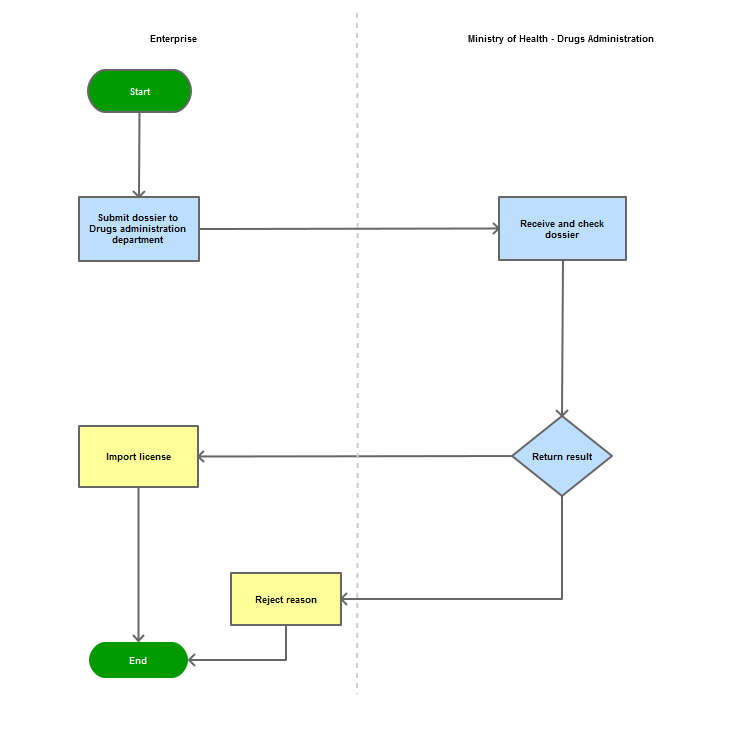
|
|---|
| Category | Procedure |
|---|
Please customize this view
This procedure apply to theses Measures.

