View Procedure
| Procedure Name | Granting permission to import chemicals and preparations without registration numbers for circulation for research, under an assistance program or used for other specific purposes (as gifts or presents or in case similar products or methods are unavailabl |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Ministry of Health - Health Environment Management Agency
Address: Alley 135 Nui Truc street, Ba Dinh district, Hanoi
Phone: +844.37368395
Email:
|
|
Legal base of the Procedure
|
- Law on Product and Goods Quality No. 05/2007/QH12 dated November 21, 2007.
- Commercial Law No. 36/2005/QH11
- Law on Chemicals No. 105/2016/QH13 dated April 6, 2016 of the National Assembly
- Decree 187/2013/ND-CP dated November 20, 2013 of the Government, detailing the Commercial Law regarding international goods purchase and sale and goods purchase and sale agency, processing and transit activities with foreign partners
- Decree 132/2008/ND-CP dated December 31, 2008, detailing the implementation of a number of articles of the Law on Product and Goods Quality
- Decree 26/2011/ND-CP of April 8, 2011, amending and supplementing a number of articles of the Government's Decree No. 108/2008/ND-CP of October 7; 2008, detailing and guiding a number of articles of the Law on Chemicals
- Decree 91/2016/ND-CP dated July 1, 2016 of the Government on management of insecticidal and germicidal chemicals and preparations for household and medical use
|
|
Processing time
|
- Within 15 working days from the date of reception of the adequate and valid dossier
|
|
Fee
|
|
Required documents:
|
No
|
Type of documents
|
Note
|
|
1
|
An application for import permit (made according to Form 2, Annex 4)
|
01 original copy
|
|
2
|
A notarized copy of the business registration certificate or other papers proving the legal entity status of the import license applicant, which must be appended with the applicant's seal
|
01 photocopy
|
|
3
|
Technical documents of chemicals or preparations
|
01 original copy
|
|
4
|
The research outline (for chemicals and preparations imported for research) or written explanations about the purposes of chemicals or preparations (for those imported for specific purposes)
|
01 original copy
|
|
5
|
A GMP or ISO certificate of the manufacturer and circulation permits of the chemicals or preparations granted by the country of origin or circulation permits granted by other countries in which the chemicals or preparations have been registered and sold, in case of importing chemicals or preparations for donation or other specific purposes with a total amount of 100 (one hundred) smallest units of packaging, posing possible risks to the communities
|
01 original copy
|
Process Steps
|
Step 1
|
The enterprise shall submit the dossier of application for import via postal service or directly to the Document Unit of the Health Environment Management Agency - Ministry of Health
|
|
Step 2
|
The Agency shall examine the dossier and reply in writing
|
|
Step 3
|
Inform the enterprise of the results
|
Process Map: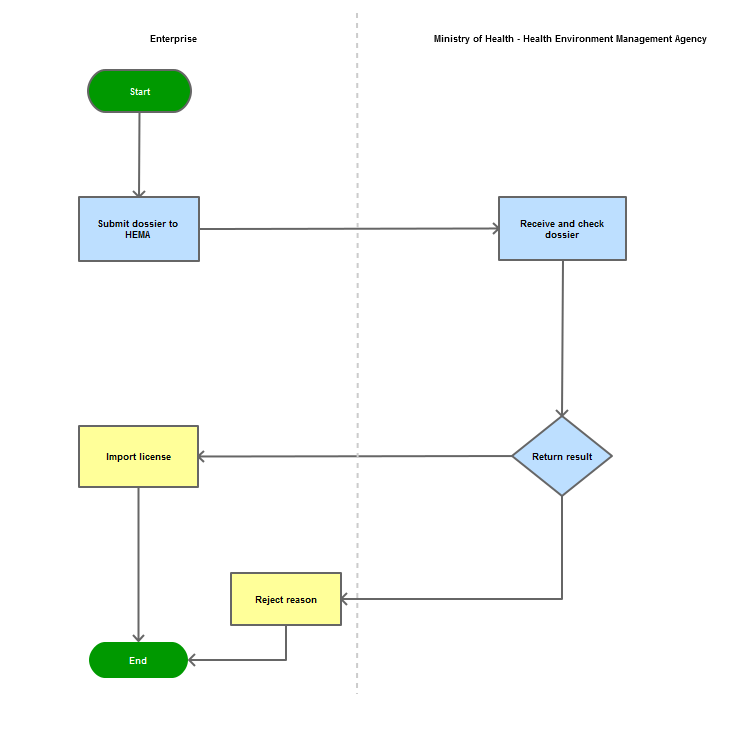
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures


