View Procedure
| Procedure Name | Granting permission to import chemicals and preparations without registration numbers for circulation in large volumes for exterminating insects or bacteria on board of aircrafts (in case similar products or methods are unavailable in the domestic market) |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Ministry of Health - Health Environment Management Agency
Address: Alley 135 Nui Truc street, Ba Dinh district, Hanoi
Phone: +844.37368395
Email:
|
|
Legal base of the Procedure
|
- Law on Product and Goods Quality No. 05/2007/QH12 dated November 21, 2007.
- Commercial Law No. 36/2005/QH11
- Law on Chemicals No. 105/2016/QH13 dated April 6 2016 of the National Assembly
- Decree 187/2013/ND-CP dated November 20, 2013 of the Government, detailing the Commercial Law regarding international goods purchase and sale and goods purchase and sale agency, processing and transit activities with foreign partners
- Decree 132/2008/ND-CP dated December 31, 2008, detailing the implementation of a number of articles of the Law on Product and Goods Quality
- Decree 26/2011/ND-CP of April 8, 2011, amending and supplementing a number of articles of the Government's Decree No. 108/2008/ND-CP of October 7; 2008, detailing and guiding a number of articles of the Law on Chemicals
- Decree 91/2016/ND-CP dated July 01, 2016, on management of insecticidal and germicidal chemicals and preparations for household and medical use
|
|
Processing time
|
- Within 05 days for assaying
- Within 10 days for informing the applicant of the results.
|
|
Fee
|
|
Required documents:
|
No
|
Type of documents
|
Note
|
|
1
|
An application for import permit (made according to Form 2, Annex 4)
|
01 original copy
|
|
2
|
A notarized copy of the business registration certificate or other papers proving the legal entity status of the import license applicant, which must be appended with the applicant's seal
|
01 photocopy
|
|
3
|
Technical documents of chemicals or preparations
|
01 original copy
|
|
4
|
A GMP or ISO certificate of the manufacturer and circulation permits of the chemicals or preparations granted by the country of origin or circulation permits granted by other countries in which the chemicals or preparations have been registered and sold
|
01 original copy
|
|
5
|
The assay result slip (supplemented to the dossier after the import license applicant receives it from the assaying unit)
|
01 original copy
|
Process Steps
|
Step 1
|
The enterprise shall submit the dossier of application for import permit via postal service or directly to the Document Unit of the Health Environment Management Agency - Ministry of Health
|
|
Step 2
|
Within 5 (five) working days, the Ministry of Health (the Health Environment Management Agency) shall issue a written request for assay
|
|
Step 3
|
Within 10 working days after receiving the assay result notice from the import license applicant, the Ministry of Health (the Health Environment Management Agency) shall issue a document stating its acceptance or rejection of the import. In case of rejection, relevant reasons shall be clearly stated
|
|
Step 4
|
Inform the enterprise of the results
|
Process Map: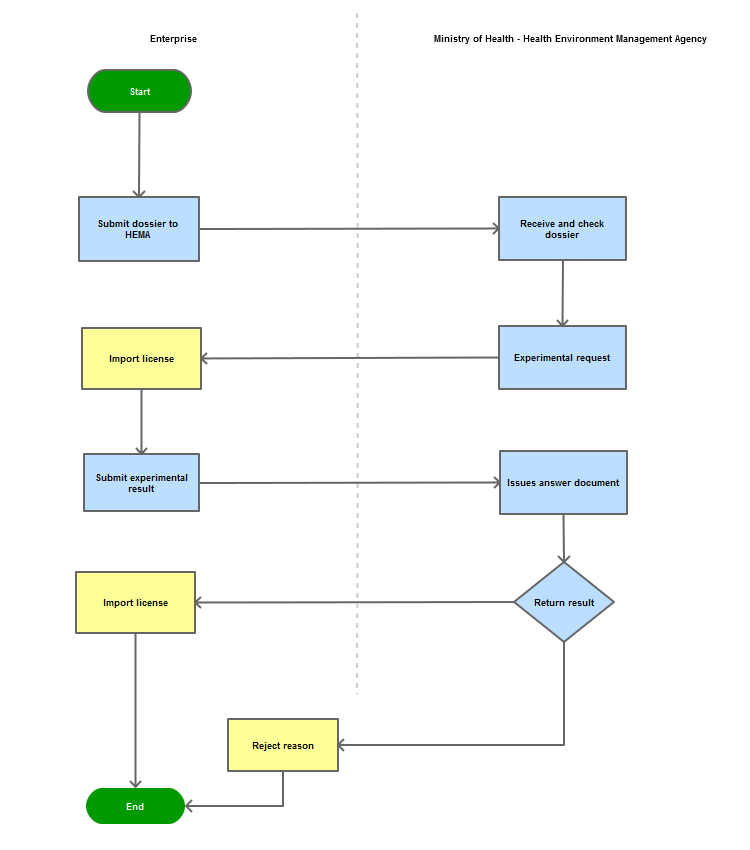
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures


