View Procedure
| Procedure Name | Granting permission to export/import patient specimens |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Ministry of Health - General Department of Preventive Medicine
Address: Alley 135 Nui Truc street, Ba Dinh district, Hanoi
Phone: +844 3843 0040
Email:
|
|
Legal base of the Procedure
|
- Circular 43/2011/TT-BYT the management of patient specimens of infectious diseases
|
|
Processing time
|
- Within 10 working days from the date of reception of the adequate and valid dossier
|
|
Fee
|
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
Dossier of application for the import or export of specimens
|
01 original copy
|
|
2
|
The documentations proving the necessity of the import or export of specimens, including the photocopy of the written approval issued by a ministerial or provincial competent authority for the research or project, and the outline of the research or project that is approved of, or the written agreement on the cooperation between a Vietnamese party and a foreign party relating to the import or export of specimens.
|
01 original copy
|
|
3
|
The photocopy of the Certificate of Business registration, Establishment Decision or other papers proving that the organization is licensed to export, import, research, preserve, transport, and test specimens.
|
01 photocopy
|
|
4
|
Where an organization licensed to research authorizes another eligible organization to import or export specimens, the dossier must contain the contract signed by both of them.
|
01 original copy
|
Process Steps
|
Step 1
|
The applicant shall submit 01 dossier set of original documents/materials to the General Department of Preventive Medicine (Department of Vaccines and Testing Management)
|
|
Step 2
|
If the dossier is complete or valid, the General Department of Preventive Medicine shall: within 10 working days from the day on which the complete and valid dossier is received, issue a written decision to grant or not to grant the Permit to import or export the patient specimens. If the dossier is incomplete or not valid, the General Department of Preventive Medicine shall within 10 working days notify such in writing to the applicant for supplementation of the dossier
|
|
Step 3
|
If the final dossier is satisfactory and eligible, the General Department of Preventive Medicine shall make the decision to grant the Permit to import or export the patient specimens. In case of rejection, relevant reasons shall be clearly stated
|
Process Map: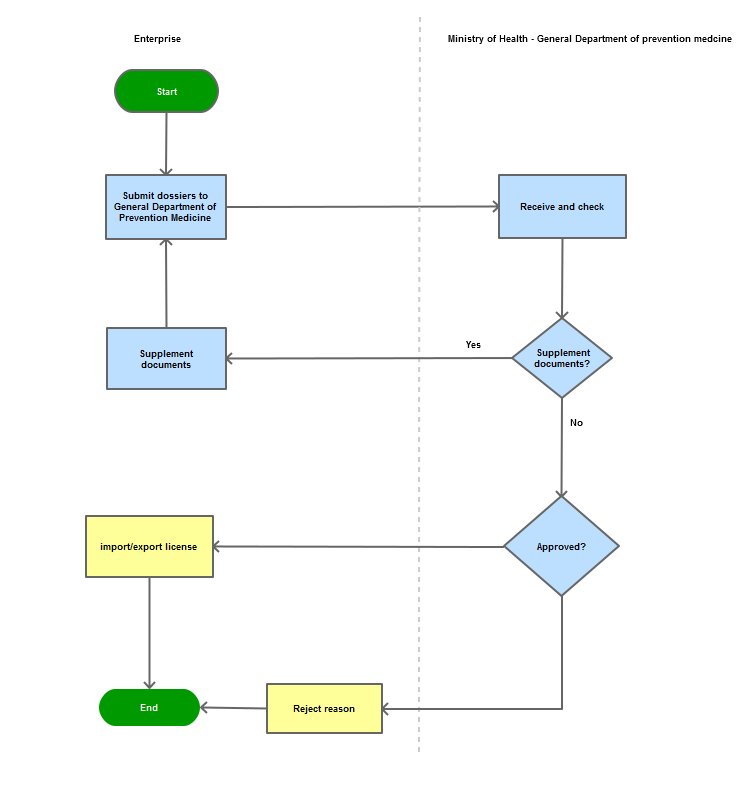
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures


