| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Ministry of Health - Health Environment Management Agency
Address: 135/1 Nui Truc – Ba Dinh - Hanoi
Phone: (+844) 3736 (ext:
Email:
|
|
Legal base of the Procedure
|
- Law on Product and Goods Quality No. 05/2007/QH12 dated November 21, 2007
- Law No. 06/2007/QH12 of November 21, 2007, on Chemicals.
- Law No. 67/2014/QH13 dated November 26, 2014, on investment
- Law No. 68/2014/QH13 dated November 26, 2014, on enterprises
- Law No. 68/2006/QH11 of June 29, 2006 on standards and technical regulations
- Decree 91/2016/ND-CP dated July 01, 2016, on management of insecticidal and germicidal chemicals and preparations for household and medical use
- Circular 277/2016/TT-BTC dated November 14, 2016, on amounts, collection, payment, management and use of fees in the fields of pharmacy and cosmetics
|
|
Process time
|
- Within 30 days, since the date received full dossier, HEMA issues document for enterprise to supplement more documents, modify dossier or permit or not allowed experimental
- Within 30 days from receiving Experimental Result and component testing from enterprise to supplement to the dossier, HEMA shall issues answer of supplement more documents or grant the circulation number or refuse to grant circulation number
|
|
Fee
|
- Fee for appraisal of the application dossiers (first submitted together with dossiers of registration for new circulation): VND 2,000,000 / dossier
- Fee for appraisal of the new circulation registration (paid when supplementing questionnaire on results of assay and results of testing of active ingredients): VND 8,000,000 / dossier
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
An application for the new circulation registration
|
01 original copy
|
|
2
|
Documents on the legal status of registered establishments, manufacturing facility
|
01 original copy
|
|
3
|
Letter of attorney for registration of circulation
|
01 original copy
|
|
4
|
The technical documentation of the proposed regimen includes the contents specified in Annex V to Decree 91/2016/ND-CP.
|
|
|
5
|
Test results of composition and content of active ingredients in preparations (supplemented with the questionnaire of test results)
|
01 original copy
|
|
6
|
Questionaire of the experimental result
|
01 original copy
|
|
7
|
Label of the composition
|
|
| 8 |
Certificate of Free Sale (applied for imported product) |
|
| 9 |
Documents and research results on safety and efficiency or recommended by the World Health Organization or the other international organizations equivalent to the use of preparations for domestic and medical use (for preparations Contains active ingredient or product form for the first time registered in Vietnam). |
|
Requirements and conditions:
|
1
|
Article 19. Requirements for registered preparations
1. The toxicity of preparations of which the registration is carried out is not classified in Class Ia or Ib according to WHO’s classification system for pesticides (or insecticidal preparations), or in Heading I or II according to the Globally Harmonized System of Classification and Labeling of Chemicals (GHS).
2. Preparations must not contain any of active ingredients in the list of active ingredients banned from use in preparations.
3. The registration of preparations which contain any of active ingredients in the list of active ingredients with restricted use in preparations shall be made within the regulated scope of use only.
4. Registered preparations must be made by a manufacturer that has declared its eligibility to produce preparations as regulated (domestic preparations) or attached with Certificate of Free Sale (for imported preparations).
|
|
2
|
Article 20. Holders of sale registration certificates
1. The following entities may be holders of sale registration certificates:
a) A domestic enterprise, or co-operative, or business household that is the owner of preparations of which the registration is made or a representative office in Vietnam of a foreign trader who is the owner of relevant preparations;
b) A domestic enterprise, or a co-operative, or a business household that applies for the registration of preparations under the authorization of the owner of such preparations;
c) A representative office of foreign trader in Vietnam that applies for the registration of preparations under the authorization of the owner of such preparations.
2. In case of additional registration of preparations, or extension of registration number, or re-issuance of Sale Registration Certificate, the holder of sale registration certificate is also the registration number holder.
3. If the owner of preparations gives permission to its authorized entity to re-authorize another entity to apply for the registration of preparations, the permitted reauthorization must be clarified in the Letter of Authorization.
4. If there are two or several entities in Vietnam apply for the registration of the same type of preparations under the authorization of the owner of that type of preparations, Ministry of Health shall only receive and settle the application submitted by the entity that comes first with a valid letter of authorization.
|
Process Steps
|
Step 1
|
Organizations and individuals submit the official circulation registration dossiers and the charge for dossier evaluation to the Health Environment Management Agency
|
|
Step 2
|
Within one month from the date of receipt of the dossier, HEMA issues document allows to carry out experimental. In case of not permitting the experimental, a written reply must be made, clearly stating the reasons of refusal. |
|
Step 3
|
Within 12 months since HEMA allows to carry out experimental, the register must supplement experimental result to supplement the dossier. If 12 months are over due, the registration dossier will be expired
|
| Step 4 |
Within 30 days from receiving the supplemented experimental result, HEMA must issues circulation number or clearly document that state out the reason. |
| Step 5 |
Return the result to the register. |
Process Map: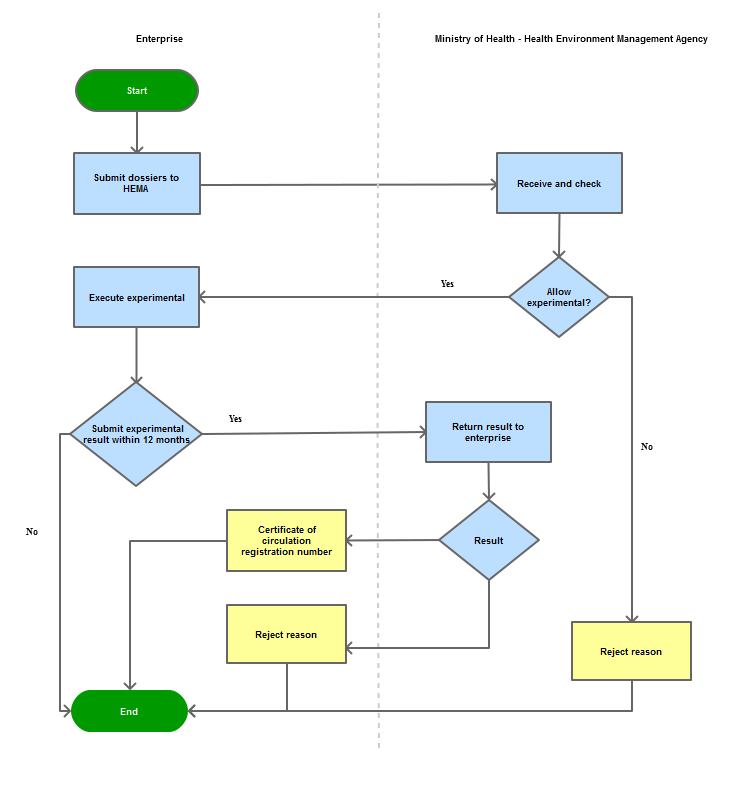
|
|---|


