View Procedure
| Procedure Name | Granting the Certificate of “Good Manufacturing Practice” (GMP) |
|---|
| Description |
|
Category
|
License/Permit
|
|
Responsible Agency
|
Drug Administration of Vietnam - Ministry of Health
Address:
Phone:
Email:
|
|
Legal base of the Procedure
|
- Law on Pharmacy No. 105/2016/QH13
- Decision No. 3886/2004/QD-BYT dated 11/3/2004 on the application of principles and standards of “good manufacturing practice" according to the recommendations of the World Health Organization
- Circular 277/2016/TT-BTC on the fees for verifying conditional lines of business; the fees for verifying the standards and conditions for medical practice and pharmacy practice
- Circular 45/2011/TT-BYT amending and supplementing a number of articles in documents regulating pharmaceutical and cosmetic industries.
|
|
Processing time
|
- Within 30 working days from the date of reception of adequate and valid dossier
|
|
Fee
|
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
A written registration for examination "Good Manufacture Practice"
|
01 original copy
|
|
2
|
A copy of establishment license or business registration certificate or investment certificate signed by the owner of the establishment and stamped for certification by the establishment
|
01 photocopy
|
|
3
|
Organizational and staffing diagram of the establishment
|
01 original copy
|
|
4
|
Data, programs, and summary reports of training and refreshing activities related to "Good Manufacture Practice" at the establishment.
|
01 original copy
|
|
5
|
Diagram of location and design of the factory, including the diagram of general plan; path diagram of the workers; path diagram of raw materials, packaging, semi-finished and finished products; diagram of water supply systems for production; gas supply diagram for the factory; diagram showing the levels of cleanliness of the factory; waste disposal diagram.
|
01 photocopy
|
|
6
|
Existing equipment list of the factory
|
01 photocopy
|
Requirements and conditions:
|
The production establishment must have at least 03 lots of products on the line registered for examination to appraise the production process and related issues such as performance evaluation of production equipment, auxiliary facilities, evaluate sanitation process; assess the capacity and the compatibility of activities of preservation, testing of drugs for the line registered for examination. These contents must be fully reflected in the profiles of lots of products and the concerned records and documents.
|
Process Steps
|
Step 1
|
The applicant shall submit a dossier of registration for GMP examination to the Drug Administration of Vietnam - Ministry of Health
|
|
Step 2
|
The Drug Administration of Vietnam shall receive and examine the dossier. Within 05 working days, the Administration shall inform the establishment on the status of dossier if it does not meet the requirements or the examination plan.
|
|
Step 3
|
Within 20 working days from the date of notification of the examination plan, the Ministry of Health shall conduct in-field examination at the establishment.
|
|
Step 4
|
The Competent Authority shall consider issuing the Certificate of "Good Manufacture Practice" or inform the registrant of official results within 05 working days after the completion of the examination or after the reception of the report on repairing actions.
|
Process Map: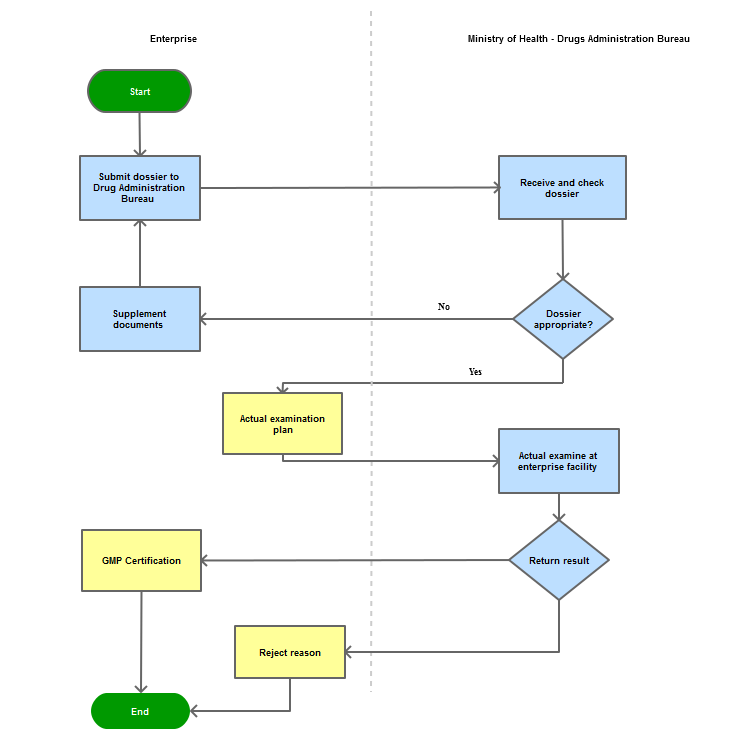
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| No results found. |


