View Procedure
| Procedure Name | Procedure for the import of veterinary drug samples used for study, testing, laboratory diagnosis, assaying, or registration for circulation |
|---|
| Description |
|
Category
|
Import permit
|
|
Responsible Agency
|
Department of Animal Health
Address: 15/78 Giai Phong, Phuong Mai, Dong Da, Ha Noi
Phone: +(844) 3869.5527/3869.6788
Email: quanlythuoc@gmail.com
|
|
Legal base of the Procedure
|
- Circular 04/2015/TT-BNNPTNT guiding the implementation of Decree 187/2013/ND-CP of the Government, detailing the Commercial Law's provisions on international goods trading and goods trading, processing and transit agency with foreign countries in the domains of agriculture, forestry and fisheries
|
|
Processing time
|
- 12 days for risk assessment
- 03 days for granting of permit after receiving risk assessments
|
|
Fee
|
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
Written application for import permit of veterinary drugs using the form No.01/TY issued together with this Circular; concurrently send the electronic file of application form (word, excel) via email: quanlythuoc@gmail.com
|
Form No. 01/TY
|
|
2
|
A copy of Certificate of Business Registration of the organization or individual applying for the permit for import of veterinary drugs
|
|
|
3
|
A Good manufacturing practice (GMP) certificate or International Organization for Standardization (ISO) certificate or equivalent standards of the manufacturer (regarding some common chemicals)
|
|
|
4
|
A Certificate of free sale issued by competent agency of the exporting country (CFS, CPP, MA)
|
|
|
5
|
A Certificate of analysis (CoA) issued by manufacturer
|
|
|
6
|
A summary of product characteristics
|
|
Process Steps
|
Step 1
|
Submit the dossier of application registration directly to the Department of Animal Health.
|
|
Step 2
|
The Department of Animal Health. shall examine the validity of the dossier and request for supplements (if any)
|
|
Step 3
|
Grant the import permit or
Reply in witting in case of refusal
|
Process Map: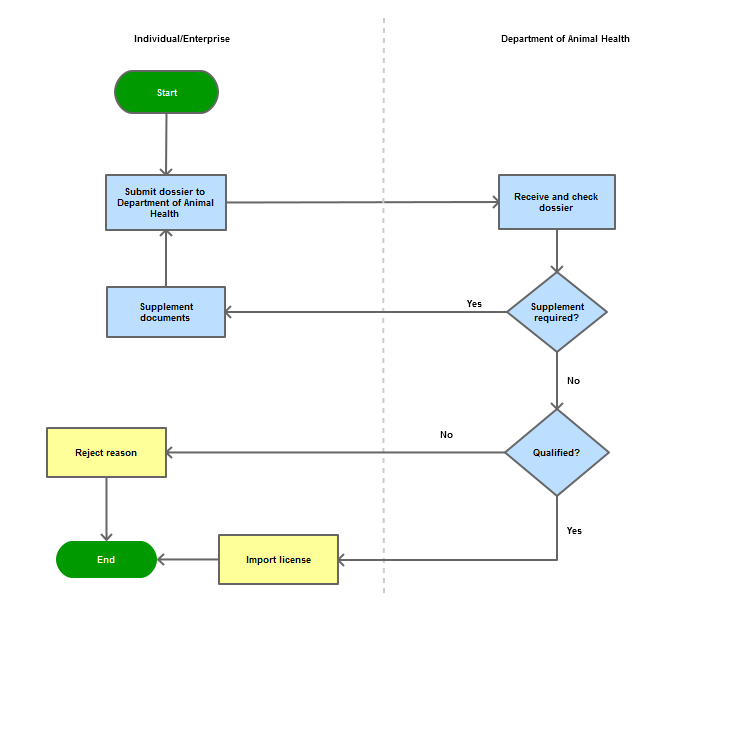
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Granting of Import Permit of veterinary drug samples used for study, testing, laboratory diagnosis, assaying or registration for circulation | Permit Requirement | Ministry of Agriculture and Rural Development | Any veterinary drug without CFS or not included in the List of veterinary drugs permitted for sale in Vietnam shall be imported in following cases: a) Materials used for manufacture of veterinary drugs with CFS; b) Samples used for study, testing, laboratory diagnosis, assaying or registration for circulation c) Chemical standards used for veterinary diagnosis or assaying veterinary drugs displayed in trade fairs, exhibitions or used for prevention and treatment for precious and rare animals; d) Medical aid of international organizations and types of other non-commercial imports; dd) Prevention and treatment of urgent epidemic diseases, disaster recovery
| Article 19, import veterinary drug for study, testing, laboratory diagnosis, assaying or registration for circulation
| Circular 04/2015/TT-BNNPTNT, on guidance on the Decree 187/2013/ND-CP on guidance on the Commercial law on international trade in goods and commercial agency, trading, processing and transit of goods with foreign countries in the Agriculture, Forestry | 31-12-9999 | Good |
143


