View Procedure
| Procedure Name | Registration for processing of veterinary drugs without certificates of free sale in Vietnam for export |
|---|
| Description |
|
Category
|
Procedure for granting certification
|
|
Responsible Agency
|
Department of Animal Health - Ministry of Agriculture and Rural Development
Address: No. 15/78 Giai Phong Rd, Phuong Mai, Dong Da, Hanoi
Phone: 04.36290447
Email: Quanlythuoc@dah.gov.vn
|
|
Legal base of the Procedure
|
- Law on Veterinary Medicine 79/2015/QH13
- Decree 35/2016/ND-CP of the Government guiding the implementation of the Law on Veterinary Medicine
- Circular 13/2016/TT-BNNPTNT On veterinary drug management
- Circular 285/2016/TT-BTC On collection, payment, management and amount of veterinary fees and charges
|
|
Processing time
|
- Within ten (10) working days, inform the registering organization of the need for completion if the dossier is not complete;
- Within twenty (20) working days from the reception of the complete and valid dossier the Department of Animal Health shall examine the dossier and grant the Certificate of Free Sale
|
|
Fee
|
- Dossier examination: 1,153,000 VND/unit of product;
- Certificate granting: 70,000 VND/certificate
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
Written registration for processing for export
|
|
|
2
|
Summary of the products' properties
|
|
|
3
|
Sample product labels and user guide sheets
|
|
|
4
|
GMP Certificate of the processing establishment
|
Original or notarized copy
|
|
5
|
Manufacturing process
|
|
|
6
|
Quality standards and testing methods
|
|
|
7
|
Certificate of Analyst (CA) of the processing establishment, Certificate of Analyst (CA) granted by the designated veterinary drug testing agency in Vietnam
|
Original copy
|
|
8
|
A written commitment of processing for export and not for sale in Vietnam
|
|
|
9
|
A written commitment of non-violation of regulations of the law on intellectual property rights
|
Annex XII Circular No. 13/2016/TT-BNNPTNT
|
Process Steps
|
Step 1
|
The processing establishment shall submit the dossier of registration for processing of veterinary drugs to the Department of Animal Health
|
|
Step 2
|
Upon receiving the dossier of registration for processing, the Department of Animal Health shall inform the registering organization of the need for completion if the dossier is not complete;
|
|
Step 3
|
Upon receiving the complete and valid dossier, the Department of Animal Health shall examine the dossier and inform the registering organization of the result
|
|
Step 4
|
Grant the Certificate of Free Sale (CFS) if the dossier is satisfactory
|
Process Map: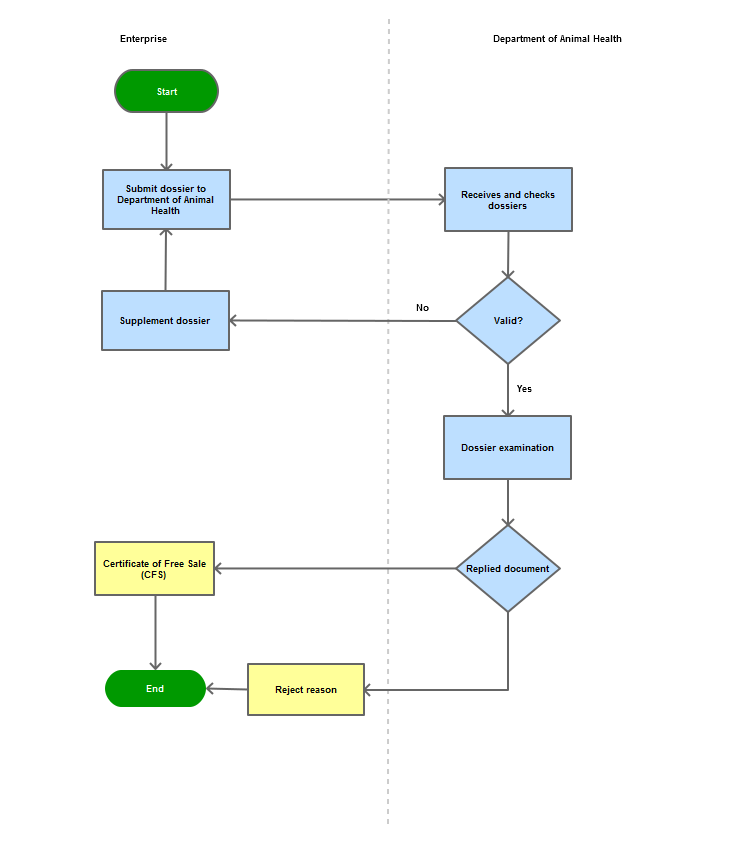
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures


