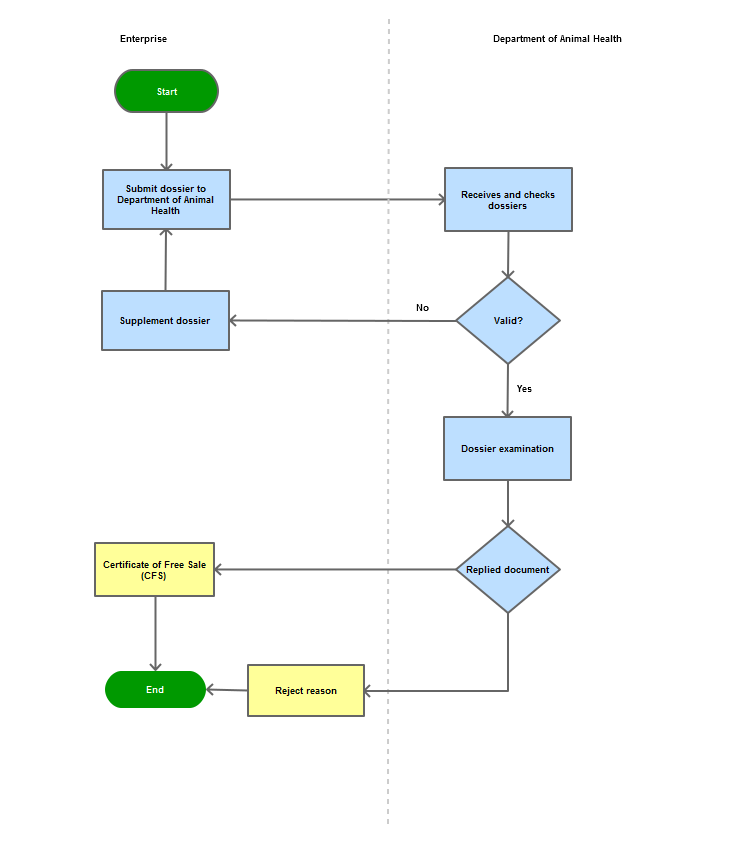
|
No.
|
Type of documents
|
Note
|
|
Import veterinary drugs for test or registration
|
|
1
|
Written registration for import of veterinary drugs (not included in the list of veterinary drugs permitted for circulation in Vietnam)
|
Form XXXII Circular No. 13/2016/TT-BNNPTNT
|
|
2
|
Certificate of Business registration or the Certificate of Enterprise registration or the investment license of the applicant
|
Copy
|
|
3
|
GMP certificate or an ISO certificate or other equivalent certificates
|
Original
|
|
4
|
Certificate of free sale (CFS, CPP or MA) issued by a competent body of the country of manufacture
|
Original
|
|
5
|
Certificate of analysis (CoA) of the manufacturer
|
Original
|
|
6
|
Sample product labels
|
|
|
|
|
Import veterinary drugs without the certificate of free sale which serving the prevention and fighting against emergency animal epidemic and/or serving disaster recovery
|
|
1
|
Written registration for import of veterinary drugs (not included in the list of veterinary drugs permitted for circulation in Vietnam)
|
Form XXXII Circular No. 13/2016/TT-BNNPTNT
|
|
2
|
Certificate of Business registration or the Certificate of Enterprise registration or the investment license of the applicant
|
Copy
|
|
3
|
GMP certificate or an ISO certificate or other equivalent certificates
|
Original
|
|
4
|
Certificate of free sale (CFS, CPP or MA) issued by a competent body of the country of manufacture
|
Original
|
|
5
|
Certificate of analysis (CoA) of the manufacturer
|
Original
|
|
6
|
Sample product labels
|
|
|
|
|
|
|
Import veterinary drugs to be displayed at a fair, an exhibition or for scientific research
|
|
1
|
Written registration for import of veterinary drugs (not included in the list of veterinary drugs permitted for circulation in Vietnam)
|
Form XXXII Circular No. 13/2016/TT-BNNPTNT
|
|
2
|
Certificate of Business registration or the Certificate of Enterprise registration or the investment license of the applicant
|
Copy
|
|
3
|
Documents proving the purposes of importing veterinary drugs
|
Copy
|
|
4
|
Certificate of analysis (CoA) by the manufacturer
|
Original
|
|
5
|
Summary of product characteristics (SmPC)
|
|
|
6
|
The model of the product label
|
|
|
|
|
|
|
Import veterinary drugs used for animals which are temporarily imported or transited through Vietnam’s territory; temporarily imported for re-export or outward processing under a contract which a foreign organization or individual
|
|
1
|
Written registration for import of veterinary drugs (not included in the list of veterinary drugs permitted for circulation in Vietnam)
|
Form XXXII Circular No. 13/2016/TT-BNNPTNT
|
|
2
|
Certificate of analysis (CoA) by the manufacturer
|
Original
|
|
3
|
Documents proving the purposes of importing veterinary drugs
|
Copy
|
|
4
|
The model of the product label
|
|
|
|
|
|
|
Import veterinary drugs and materials thereof or microorganisms serving the research and manufacture of veterinary drugs, diagnosis or test of veterinary drug
|
|
1
|
Written registration for import of veterinary drugs (not included in the list of veterinary drugs permitted for circulation in Vietnam)
|
Form XXXII Circular No. 13/2016/TT-BNNPTNT
|
|
2
|
Certificate of Business registration or the Certificate of Enterprise registration or the investment license of the applicant
|
Copy
|
|
3
|
Certificate of analysis (CoA) by the manufacturer
|
Original
|
|
4
|
Summary of product characteristics (SmPC)
|
|