View Procedure
| Procedure Name | State quality inspection of imported veterinary drugs |
|---|
| Description |
|
Category
|
Certificate
|
|
Responsible Agency
|
Department of Animal Health - Ministry of Agriculture and Rural Development
Address: No. 15/78 Giai Phong Rd, Phuong Mai, Dong Da, Hanoi
Phone: 04.36290447
Email: Quanlythuoc@dah.gov.vn
|
|
Legal base of the Procedure
|
- Law on Product and Goods Quality No. 05/2007/QH12
- Circular 13/2016/TT-BNNPTNT dated June 2, 2016 of the Ministry of Agriculture and Rural Development on veterinary drug management
- Circular 285/2016/TT-BTC on the collection, remittance, management and amount of veterinary fees and charges
|
|
Processing time
|
- 05 working days for raw materials for veterinary drugs medicines, chemicals, biological preparations after taking samples for quality inspection
- 14 working days for vaccines and antibodies subject to inspection of sterility, purity or safety criteria after taking samples for quality inspection
- 60 working days for vaccines and antibodies subject to inspection of effectiveness criteria after taking samples for quality inspection
|
|
Fee
|
- According to Annex 6 enclosed with Circular No. 04/2012/TT-BTC
|
Required Documents
|
No.
|
Type of documents
|
Note
|
|
1
|
Written registration for quality inspection of the imported veterinary drugs
|
Annex XXXVII Circular No. 13/2016/TT-BNNPTNT (2 copies)
|
|
2
|
Contract, Packing list, Invoice, Bill of Lading
|
Copy certified by the Enterprise
|
|
3
|
Written permission to import of raw materials for veterinary drugs, vaccines or microorganisms for veterinary medicines issued by the Department of Animal Health or Certificate of Free Sale of the veterinary medicines in the form of vaccines or microorganisms
|
Original or Copy certified by the Enterprise
|
|
4
|
Certificate of Analyst (CoA) of the manufacturer
|
Original or Copy certified by the Enterprise
|
|
5
|
Sample labels of the veterinary drugs and extra-labels if the main ones fail to specify the required contents
|
Original or Copy certified by the Enterprise
|
Process Steps
|
Step 1
|
The Enterprise shall submit a dossier of registration for quality inspection to the Department of Animal Health
|
|
Step 2
|
The Department shall certify the Registration for quality inspection for settlement of procedures for customs clearance or request for completion if the dossier is not sufficient.
|
|
Step 3
|
If testing samples are not required, the Enterprise shall follow procedures for customs clearance at the border gate.
If testing samples are not required, the Enterprise shall transport the commodity to the inspection location specified in the Registration Form
|
|
Step 4
|
Within 2 days, the Department shall examine the dossier, conduct external inspection and take samples for quality inspection
|
|
Step 5
|
Issue a Notice of Testing Results if the prescribed requirements for customs clearance are met
Within 05 days, if the unsatisfactory results are not subject to any objections or complaints by the Enterprise, the inspection agency may request competent agencies for prescribed steps of treatment
|
Process Map: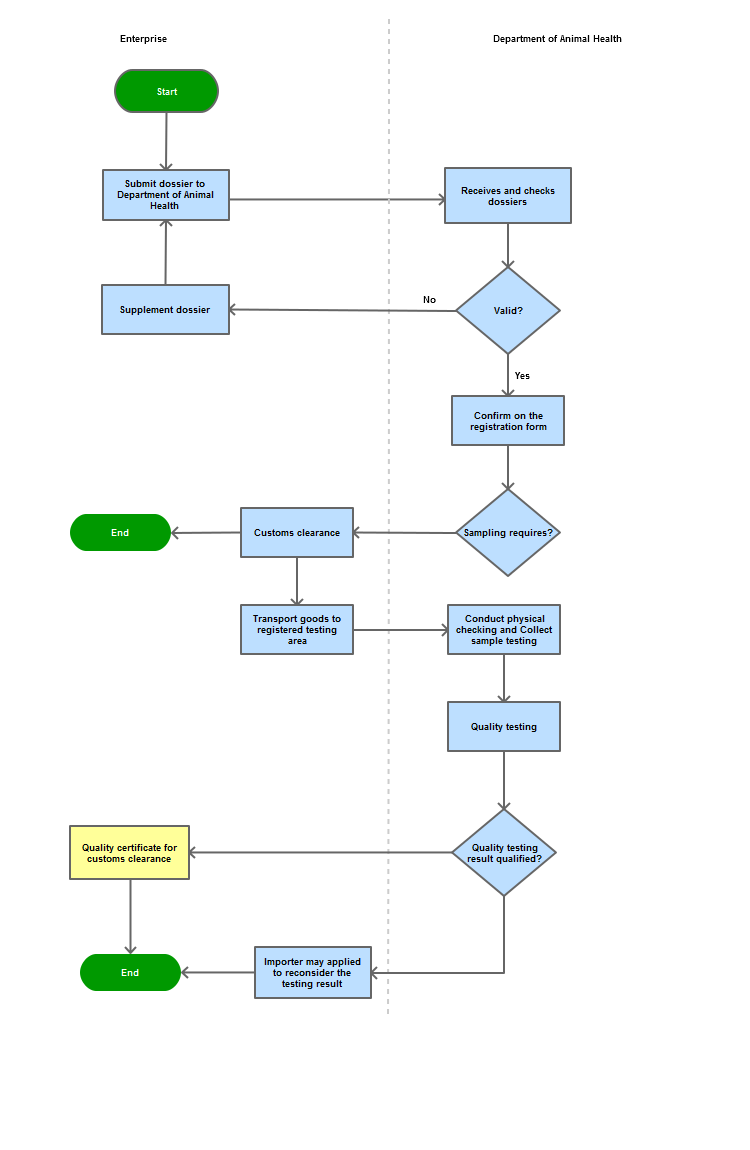
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| State quality inspection of imported veterinary drugs | Inspection Requirement | Ministry of Agriculture and Rural Development | Veterinary drugs imported into Vietnam are subject of quality test, except import for: samples for testing, registration, samples to be displayed at a fair, exhibition or a scientific research; veterinary drugs to be used for animals temporarily imported or transited through Vietnam; veterinary drugs temporarily imported for re-export or for export processing under a contract with a foreigner; materials used in diagnosis or testing pertaining to animal healthcare; drugs as aids from international organizations and drugs imported in other non-commercial forms
| Frequency of sampling: For veterinary drug's materials and finished veterinary drugs (excluding vaccines/antibodies used in animal healthcare). Collect samples of 02 batches of consecutive import shipments for quality test. If the batches are satisfactory, the frequency of sampling shall be reduced to 1 imported batch for the 05 next imported batches For vaccines/antibodies used in animal healthcare. Collect samples of every batches of imported vaccines for quality test regarding the sterility or purity and safety; collect samples from 01 batch of imported drug of 05 consecutive batches of imported drugs manufactured by the same manufacturer and imported by the sample unit for quality test regarding the effect of drugs Particularly regarding vaccines against Avian influenza, Foot-and-mouth disease, Porcine reproductive and respiratory syndrome (PRRS), 100% of batches of imported drugs shall be sampled for testing regarding the sterility, purity, safety and effect
| Circular 13/2016/TT-BNNPTNT dated June 2, 2016 on veterinary drug management | 31-12-9999 | Good |
208


