View Procedure
| Procedure Name | Cosmetic product proclamation |
|---|
| Description |
|
Category
|
Proclamation receipt number
|
|
Responsible agency
|
Drug Administration of Vietnam – Ministry of Health
Address: 138A Giang Vo Str, Ba Dinh, Hanoi
Phone: +844 3736 6483
|
|
Base on legal documents
|
- Circular 278/2016/TT-BTC Providing for fees in medical sector, and the collection, transfer, management and the usage
- Circular 06/2011/TT-BYT Providing cosmetic management
|
|
Processing time
|
- 03 working days after receiving valid dossiers
|
|
Fee
|
|
Required documents
|
Serial
|
Document title
|
Comment
|
|
1
|
Cosmetic product proclamation report (2 copies) with proclamation data (soft copy)
|
|
|
2
|
Copy of business registration certificate of organizations, individuals who are responsible for circulation products into the market (with the enterprise’s signature and seal).
|
|
|
3
|
Original or notarized copy of letter of attorney from the producers or the owners of products authorized for organizations, individuals are responsible of putting products on the market in Vietnam (applied to the import or domestic cosmetic of which organizations or individuals are responsible of putting products on the market, be not the manufacturer). For the import product, the letter of attorney must be a copy notarized sign and consul legalized as provisions of law, except for being exempted of the consult legalization in regard to international treaties in which Vietnam is a member. The letter of attorney must satisfy requirements regulated at the Article 6 of Circular 06/2011/TT-BYT
|
|
|
4
|
Certificate of free sale (CFS) is only applied for import cosmetic product proclamation which satisfies the following requirements:
a. CFS which is issued by the current territory must have been original or legally notarized and still in the day of validity. In case CFS is not provided of the expiry day, it must be a certificate which has just been issued within 24 months.
b. CFS must be consul legalized according to provisions of the law, except consul legalization immunity case according to the international treaties in which Vietnam is a member.
|
|
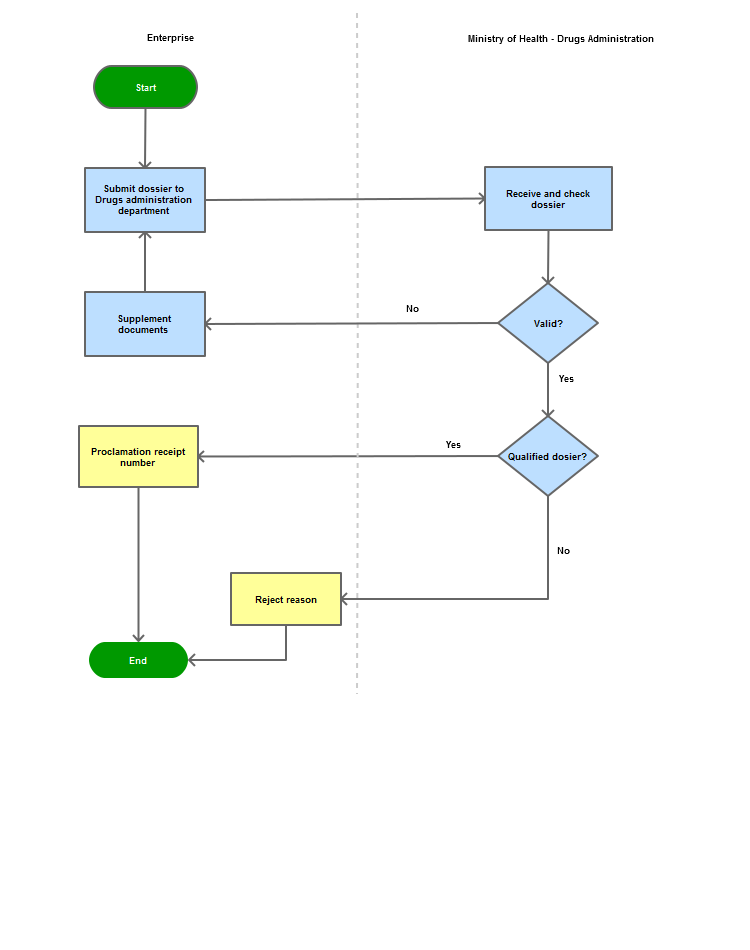
Process steps:
|
Step 1
|
Submit dossier to DAV
|
|
Step 2
|
Receives and verifies
|
|
Step 3
|
Supplement dossier if requires
|
|
Step 4
|
Issues the proclamation receipt number or the reason of the denial
|
Process map:
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Import of cosmetics requires to have cosmetic product proclamation | Technical Measures | Ministry of Health | Organizations or individuals which are responsible of putting the cosmetic product on the market just permitted selling cosmetic when be issued the number of cosmetic product proclamation receiving by the authority agencies as well as responsible for safety, effectiveness, and quality of product. The authority agencies shall carry out after-sales inspection when the product has been being sold in the market
| Organizations or individuals who are responsible for putting the products on the market must have the function of cosmetic business in Vietnam.
Cosmetic product feature proclamation (the cosmetic usage purpose) must satisfy the ASEAN’s instruction of the cosmetic product feature proclamation (Appendix No 3-MP). | Circular 06/2011/TT-BYT Regulation on management of cosmetics | 31-12-9999 | Good |
286