View Procedure
| Procedure Name | Insecticide and disinfectant official circulation registration for household and medical use |
|---|
| Description |
|
Category
|
Registration
|
|
Responsible agency
|
Medical environmental management bureau – Ministry of Health
Address:
Phone:
Email:
|
|
Base on legal documents
|
- Law on Product and Goods Quality 05/2007/QH12 dated November 21, 2007.
- Commercial Law 36/2005/QH11
- Law on Chemicals 06/2007/QH12 dated November 21, 2007 of the National Assembly
- Decree 187/2013/ND-CP dated November 20, 2013 of the Government, detailing the Commercial Law regarding international goods purchase and sale and goods purchase and sale agency, processing and transit activities with foreign partners
- Decree 132/2008/ND-CP dated December 31, 2008, detailing the implementation of a number of articles of the Law on Product and Goods Quality
- Decree 26/2011/ND-CP dated April 8, 2011 of the Government amending and supplementing a number of articles of Decree 108/2008/ND-CP dated October 7, 2008 of the Government detailing and guiding the implementation of a number of articles of the Law on Chemicals
- Decree 91/2016/ND-CP On management of insecticidal and germicidal chemicals and preparations for household and medical use
- Circular 278/2016/TT-BTC Providing for fees in medical sector, and the collection, transfer, management and the usage
|
|
Processing time
|
|
|
Fee
|
|
Required documents
|
Serial
|
Title
|
Comment
|
|
1
|
A written request for official circulation registration
|
Form 4 Annex 1
|
|
2
|
The registrant's business registration certificate, investment certificate or establishment license, for registrants being representative offices
|
|
|
3
|
The paper of authorization to carrying out circulation registration if Vietnam-based organizations and individuals that produce or trade in chemicals or preparations or Vietnam-based permanent representative offices of foreign enterprises which are authorized by chemical or preparation owners to carry out the registration
|
Original
|
|
4
|
Results of testing of the composition and contents of active ingredients of the chemical or preparation
|
|
|
5
|
The assay result slip
|
|
|
6
|
The producer's written environmental protection commitment or decision issued by a competent state agency approving the producer's environmental impact assessment report
|
|
|
7
|
Technical documents of the chemical or preparation requested for registration 9 (covering the details specified in Annex V Decree 91/2016/ND-CP)
|
|
|
8
|
The specimen label and its content registered for circulation in Vietnam
|
|
|
9
|
A valid certificate of free sale granted by a competent authority of a country permitting the circulation of the chemical or preparation requested for registration
|
|
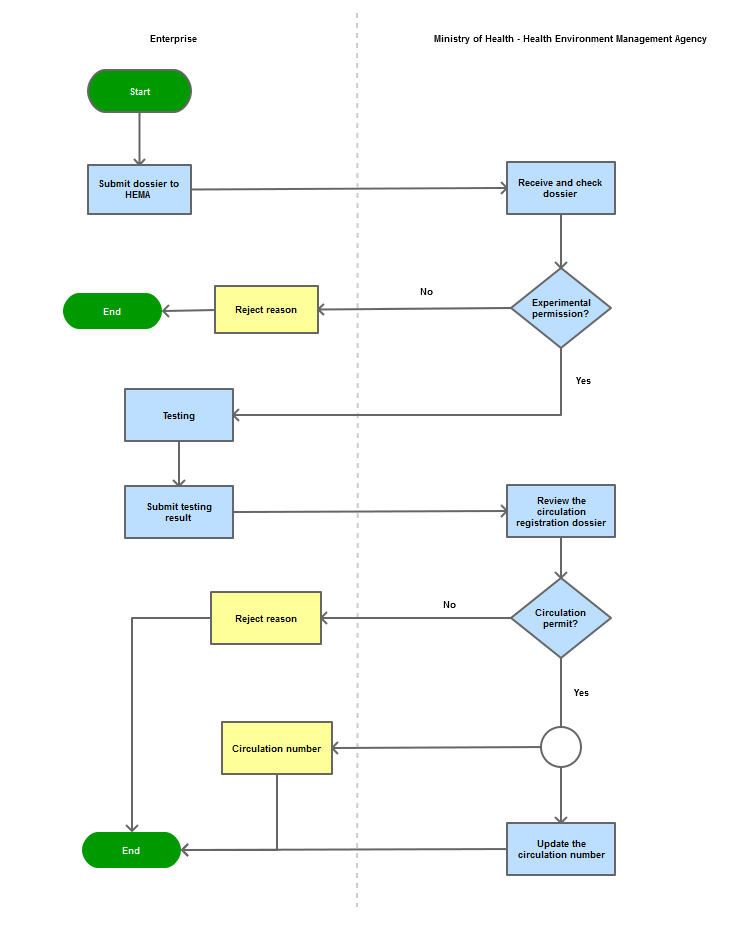
Process steps:
|
Step 1
|
The enterprise shall submit the dossier of application for import via postal service or directly to the Document Unit of the Health Environment Management Agency - Ministry of Health
|
|
Step 2
|
Within 01 month, MoH (HEMA) issues permit license for experimental/test or reject reason
|
|
Step 3
|
Within 12 months, enterprise submit test result for supplementing circulation registration dossier
|
|
Step 4
|
Within 20 days after receiving test result, MoH (HEMA) issues circulation number or reject reason
|
|
Step 5
|
Return circulation number to enterprise
|
Process map:
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| Circulation registration for chemicals and preparations | Technical Measures | Ministry of Health | Preparations which are permitted to use abroad but imported to use in Vietnam for the first time must register for circulation | Additional registration of preparations shall apply to preparations whose registration number has been issued in Vietnam and is still in effect but there is one of the following changes occurs:
a) Change of the ownership of the registration number;
b) Change of the trade name of relevant type of preparations;
c) Change of manufacturer’s location or change of manufacturer;
d) Change of name or contact address of the entity carrying out the registration, or change of name of manufacturer or production location;
dd) Change of effects, quality criteria or method of using preparations | Decree No. 103/2016/ND-CP dated July 01, 2016 of the Government on Biosafety in Laboratories | 01-07-2016 | Good |
289