View Procedure
| Procedure Name | Procedure for registration of circulation of insecticidal or germicidal chemicals and preparations for household and medical use |
|---|
| Description |
|
Category
|
Circulation Registration
|
|
Responsible agency
|
Health Environment Management Agency – Ministry of Health
Address: Ngo 1/153 Nui Truc – Ba Dinh - Hanoi
Phone: +844 3736 8395
Email:
|
|
Base on legal documents
|
- Law No. 05/2007/QH12 on product and goods quality.
- Chemicals Law No. 06/2007/QH12
- Commercial Law No. 36/2005/QH11
- Law on standards and technical regulations No. 68/2006/QH11
- Decree 91/2016/ND-CP on management of insecticidal and germicidal chemicals and preparations for household and medical use
- Circular 278/2016/TT-BTC providing for fees in medical sector, and the collection, transfer, management and usage
|
|
Processing time
|
- No later than 30 days to answer for approval or rejection of experimental allowance.
- No later than 30 days after receiving experimental result to issue Circulation number or not.
|
|
Fee
|
- Dossier evaluation for experimental fee (submit together with the new registration dossier: 2.000.000 VND/Dossier
- New circulation registration fee: 8.000.000 VND/Dossier
|
Required documents
|
Serial
|
Document title
|
Comment
|
|
1
|
Application for registration of official circulation. Form 04 Decree 91/2016/ND-CP
|
01 Original
|
|
2
|
Business registration certificate, investment certificate or establishment license.
|
01 bản sao
|
|
3
|
Power of attorney for registration of circulation except for cases specified at Point a, Clause 20 Decree 91/2016/ND-CP
|
01 bản chính
|
|
4
|
Technical documents of chemicals or preparations including content specified at Annex V Decree 91/2016/ND-CP
|
01 bản chính
|
|
5
|
Test results of composition and content of active ingredients in the composition (to be added together with the experimental results).
|
01 bản chính
|
|
6
|
Experimental result (To be supplemented after the MoH issues a document permitting testing).
|
01 bản chính
|
|
7
|
Sample label of the composition
|
01 bản sao có đóng dấu của đơn vị đăng ký
|
|
8
|
Certificate of Free Sale (for imported composition).
|
01 bản sao công chứng
|
|
9
|
Documents, research result on safety and effectiveness or recommendation of the World Health Organization or equivalent international organizations on the usage of composition in the field of home and medical (For preparations containing the active ingredient or product form for the first time registered in Vietnam).
|
|
Conditions for procedure execution
|
1
|
Documents in foreign languages must be translated into Vietnamese and be accompanied by original documents in foreign languages. Documents in foreign languages which are not English must be translated into Vietnamese with the notary's or translators'
|
|
2
|
Conditions for preparations for circulation registration:
Toxicity of the preparation is not classified in class Ia, Ib according to World Health Organization classification for insecticidal or Group I, II according to the classification of Globally Harmonized System - GHS
Does not contain active ingredients in the list of prohibited in use in preparations
Compositions containing active ingredients on the list of limited scope of usage in the preparations are only be registered for circulation with the prescribed scope of use.
Produced at the facility has announced qualified for production (for domestically manufactured preparations) or have a certificate of free sale (for imported products).
|
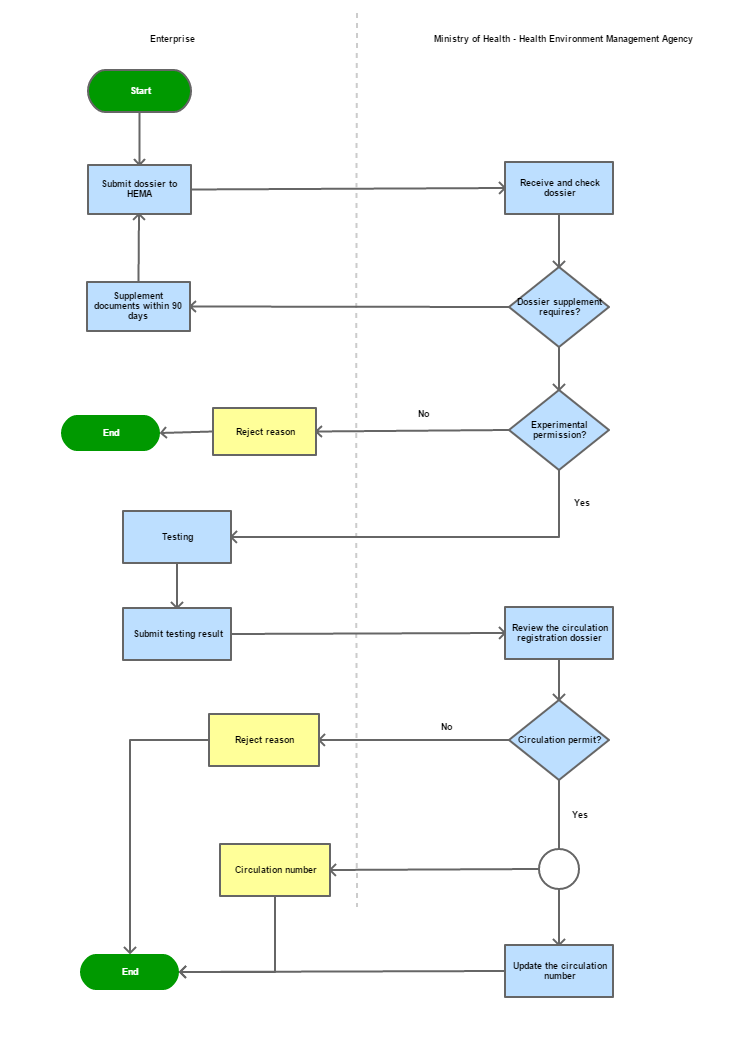
Process steps:
|
Step 1
|
Enterprises applying for circulation of insecticidal and germicidal preparations for domestic and medical use shall submit dossiers to the Health Environment Management Agency (HEMA), Ministry of Health
|
|
|
Step 2
|
After receiving dossier, HEMA sends back a Dossier Receipt (Form 04 Annex III Decree 91/2016/ND-CP
|
|
|
Step 3
|
Within 30 days from the date of sending Dossier Receipt HEMA issues dossier supplement request, experimental allows/rejects.
In case of supplement: Within 90 days must complete the documents and detail explaination of the supplemented contents.
|
|
|
Step 4
|
Within 12 months since HEMA issues document that allow the experimental, enterprises must submit test result to supplement the circulation registration. After the above said time expires, the sumitted dossier will no longer be valid for circulation registration.
|
|
|
Step 5
|
Within 30 working days since the experimental test result submitted to supplement to the dossier, HEMA will issues circulation number or return the reason why it was rejected.
|
|
|
Step 6
|
Return the result to enterprise who register for circulating.
|
|
Process map:
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures