View Procedure
| Procedure Name | Licensing for import of pharmaceuticals not used as samples for drug testing and research, display at exhibitions, fairs or production of drugs for export or manufacture of drugs in service of national defense or security requirements, prevent epidemics, |
|---|
| Description |
Required documents
|
Item
|
Required document
|
|
|
1
|
03 original copy of the purchase order according to Form No 41 in Appendix III Decree 54/2017/NĐ-CP
|
|
2
|
Documents proving that quality standards of the herbal ingredient are conformable with the National Technical Regulation on herbal ingredients according to Vietnam’s pharmacopoeia or a foreign pharmacopoeia recognized by the Ministry of Health.
If the National Technical Regulation on the herbal ingredient is not available in Vietnam’s pharmacopoeia or a foreign pharmacopoeia recognized by the Ministry of Health, the applicant shall provide quality standards including the testing method which has been evaluated by a state-owned testing laboratory;
|
|
3
|
Certified true copy of the certificate of registration of the representative office in Vietnam of the foreign exporter or the license for pharmacy business of the state-owned enterprise in Vietnam which is licensed to trade in herbal ingredients, prepared and processed herbal ingredients
|
|
4
|
Certified true copy of the exporter’s business license which allows export of herbal ingredients and is issued by a competent authority of the exporter’s home country;
|
|
5
|
Certified true copy of the manufacturer’s certificate of GMP which allows production of herbal ingredients and is issued by a competent authority of the manufacturer’s home country
|
|
6
|
A copy bearing the importer’s seal of the manufacturer’s document authorizing the foreign exporter to export herbal ingredients, unless the manufacturer is also the exporter. The content of such document is specified in Clause 15dd Article 91 Decree 54/2017/NĐ-CP.
|
Process Steps
|
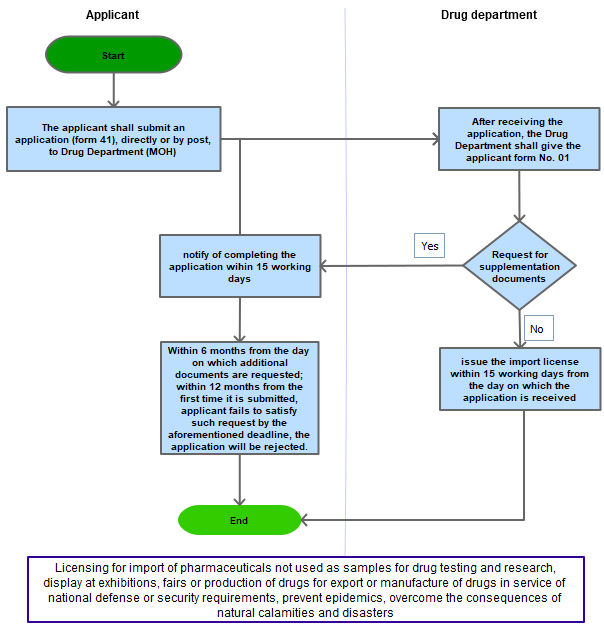
Step 1
|
The applicant shall submit an application to the Ministry of Health directly or by post
|
|
Step 2
|
After receiving the application, the Ministry of Health shall give the applicant form No. 01 in Appendix I Decree 54/2017/NĐ-CP
|
|
Step 3
|
If the application is satisfactory, the Ministry of Health shall issue the written permission to import within 15 working days from the day on which the application is received;
If the application is not satisfactory, the Ministry of Health shall request the applicant in writing to complete it within 15 working days from the day on which it is received;
|
|
Step 4
|
After receiving the supplemented application, the Ministry of Health shall give the applicant form No. 01 in Appendix I enclosed herewith. If the application is still unsatisfactory, the Ministry of Health shall request the applicant to complete it in accordance with Point d this Article. If the supplemented application is satisfactory, the Ministry of Health shall issue the written permission to import in accordance with Point c of this Clause;
Within 06 months from the day on which additional documents are requested in writing by the Ministry of Health, the applicant shall submit additional documents as requested. If the applicant fails to satisfy such request by the aforementioned deadline or the application is not satisfactory within 12 months from the first time it is submitted, the application will be rejected
|
Conditions:
- Imported drugs not used as samples for drug testing and research, exhibiting at exhibitions, fairs or production of drugs for export or production of drugs in service of national defense or security requirements or epidemic prevention and combat. disease, overcoming consequences of natural disasters, disaster.
- Importing enterprises are establishments which have been granted certificates of eligibility for pharmaceutical business, the scope of export or import of pharmaceutical materials or production thereof.
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| No results found. |


