View Procedure
| Procedure Name | Licensing for import of drugs as emergency aid or humanitarian aid |
|---|
| Description |
|
Category
|
Permission
|
|
Responsible Agency
|
MOH
|
|
Legal basis of the Procedure
|
Law 105/2016/QH13
Decree 54/2017/NĐ-CP (Article 72)
|
|
Processing time
|
within 60 days if clinical documents and documents proving equivalence to reference biologicals are not required, or within 90 days if clinical documents and documents proving equivalence to reference biological are required.
|
|
Fee
|
|
Required documents
|
Item
|
Required document
|
|
1
|
A written request for permission to import prepared by the importer and enclosed with the list of drugs to be imported as emergency aid or humanitarian aid according to Form No. 24, 25 or 26 in Appendix III Decree 54/2017/NĐ-CP
|
|
2
|
The original copy of the aid recipient’s document specifying the quantity of each type of drugs received as emergency aid or humanitarian aid and the commitment to use the drugs for intended purposes;
|
|
3
|
The original copy or certified true copy of the written approval issued by a competent authority for use of the drug for the health program of the State if the drug is provided through such a program
|
|
4
|
The original copy or a certified true copy of the certificate of pharmaceutical product
|
|
5
|
Quality documents according to regulations of the Minister of Health on use of ACTD for drug registration
|
|
6
|
Clinical document if required by regulations of the Minister of Health on use of ASEAN Common Technical Dossier (ACTD) for drug registration;
|
|
7
|
The original copy of 01 set of specimens of the label and package insert of the drug licensed for free sale in the country that issues the certificate of pharmaceutical product, unless they are already attached to the certificate of pharmaceutical product;
|
|
8
|
02 sets of specimens of the secondary label and package insert in Vietnamese language which bear the importer’s seal
|
|
9
|
A copy (authenticated or bearing the exporter’s seal) of the importer’s license to perform radiological works in case of import of radiopharmaceuticals. If a copy bearing the importer’s seal is submitted, the original copy shall be produced for comparison when the application is submitted.
|
Process Steps
|
Step 1
|
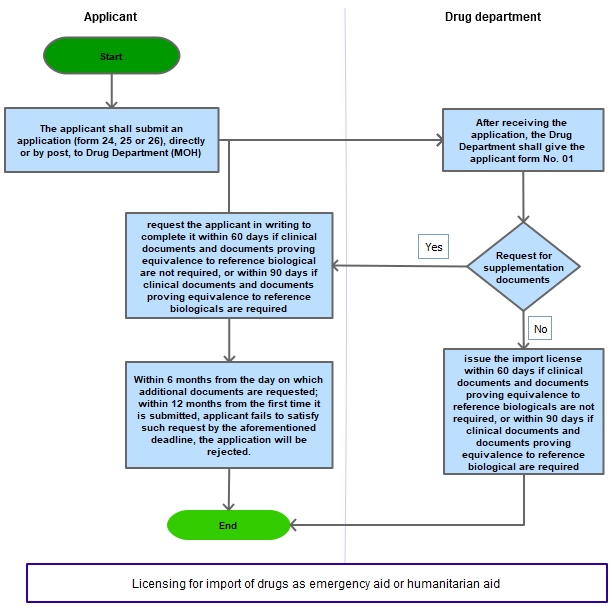
The applicant shall submit an application to the Ministry of Health directly or by post
|
|
Step 2
|
After receiving the application, the Ministry of Health shall give the applicant form No. 01 in Appendix I Decree 54/2017/NĐ-CP
|
|
Step 3
|
If the application is satisfactory, the Ministry of Health shall issue the import license within 60 days if clinical documents and documents proving equivalence to reference biologicals are not required, or within 90 days if clinical documents and documents proving equivalence to reference biological are required. The import license shall be issued on the basis of counsel given by the certification advisory council;
If the application is not satisfactory, the Ministry of Health shall request the applicant in writing to complete it within 60 days if clinical documents and documents proving equivalence to reference biological are not required, or within 90 days if clinical documents and documents proving equivalence to reference biologicals are required;
|
|
Step 4
|
After receiving the supplemented application, the Ministry of Health shall give the applicant form No. 01 in Appendix I enclosed herewith. If the application is still unsatisfactory, the Ministry of Health shall request the applicant to complete it in accordance with Point d this Article. If the supplemented application is satisfactory, the Ministry of Health shall issue the import license.
Within 06 months from the day on which additional documents are requested in writing by the Ministry of Health, the applicant shall submit additional documents as requested. If the applicant fails to satisfy such request by the aforementioned deadline or the application is not satisfactory within 12 months from the first time it is submitted, the application will be rejected
|
Conditions:
The import of such a drug shall only be licensed when the following requirements are satisfied:
b) The drug is licensed in the manufacturing country, a reference country that is a member state of the ICH or Australia;
b) The drug meets the need of the aid recipient;
c) The drug is not a narcotic drug, radiopharmaceutical or vaccine
|
|---|
| Category | Procedure |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
| No results found. |


