|
Processing of records
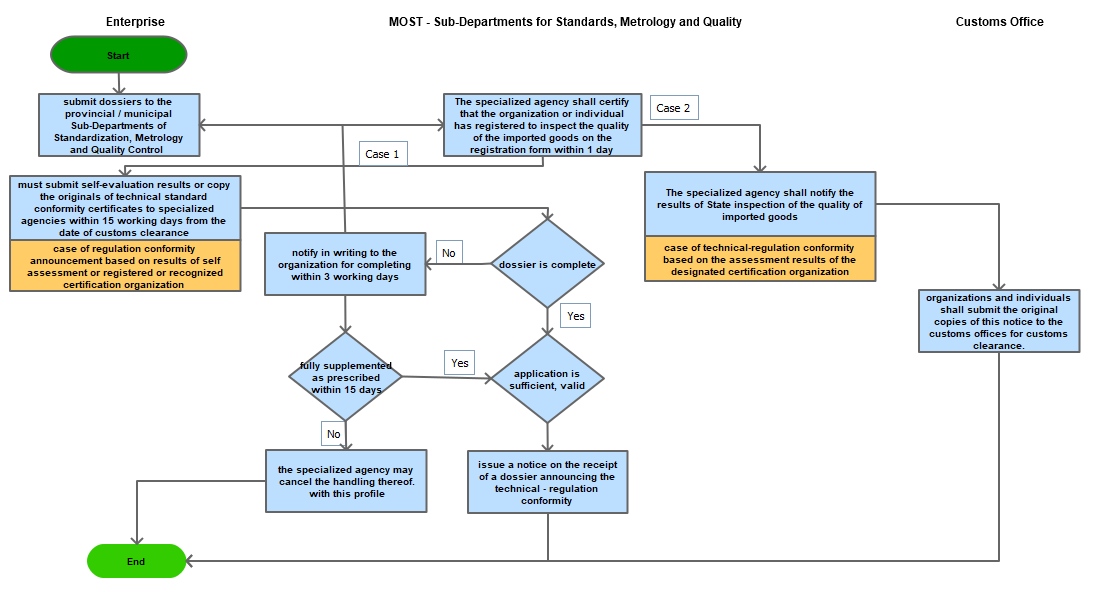
* In case of regulation conformity announcement based on results of self assessment or registered or recognized certification organization (hereinafter referred to as certification organization):
- Within one working day, the specialized agency shall certify that the organization or individual has registered for quality inspection of the import goods on the registration form of the organization or individual.
- Within 15 working days from the date of customs clearance, organizations and individuals must submit self-evaluation results according to regulations or copy the originals of technical standard conformity certificates to specialized agencies. .
* For the case of technical-regulation conformity based on the assessment results of the designated certification organization:
- The specialized agency shall notify the results of State inspection of the quality of imported goods;
- After receiving the notice on the results of the State inspection of the quality of imported goods, the organizations and individuals shall submit the original copies of this notice to the customs offices for customs clearance.
The provincial / municipal Services of Standardization, Metrology and Quality Control shall receive and check the completeness of their dossiers in the following order:
- If the dossier for registration of regulation conformity announcement is incomplete according to regulations, within 3 (three) working days after receiving the dossier of registration of regulation conformity announcement, the provincial / municipal Sub-department of Standardization, Metrology and Quality Control (hereinafter referred to as the Branch for short) shall notify in writing to the organization or individual announcing the technical-regulation conformity according to regulations. After 15 (fifteen) working days from the date of sending the written request, the dossier for registration of technical - regulation conformity notice shall not be fully supplemented as prescribed, and the specialized agency may cancel the handling thereof. with this profile.
- If the dossier for registration of technical - regulation conformity announcement is complete and valid, the branch shall issue a notice on the receipt of a dossier of registration of technical - regulation conformity announcement to the organization or individual announcing the technical - regulation conformity.
- In case the dossier for registration of technical - regulation conformity announcement is complete but invalid, the Sub - Department shall notify in writing the organization or individual of the technical - regulation conformity notice on the reasons for non - acceptance of the dossier.
|